2022 ASCO: Top 10 most anticipated breast cancer research
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
2022 ASCO: Top 10 most anticipated breast cancer research
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
2022 ASCO: Top 10 most anticipated breast cancer research.
There are still two weeks before the 2022 ASCO, and presumably many studious people have already started to preview in advance.
Dr. Erika Hamilton, director of the Breast and Gynecology Research Program at the Sarah Cannon Institute, shared her top 10 breast cancer studies on Twitter, along with her reasons for making the list.
It’s very interesting, and the cube is organized and shared here. give everyone.

NO.1: Randomized Phase III Clinical Trial TROPiCS-02 Study:
Evaluating the Efficacy of Sacituzumab govitecan vs. Doctor’s Choice of Treatment in Patients with HR+/HER2- Advanced Breast Cancer
Speaker: Hope Rugo Abstract number: LBA1001
Why it’s on the list: Could registration data in HR-positive breast cancer add another indication to goxatuzumab in addition to triple-negative breast cancer?
Med Supplementary Information:
On March 7, 2022, Gilead announced that a Phase III clinical trial of goxatuzumab in patients with HR+/HER2- metastatic breast cancer who had received multiple prior therapies met the primary endpoint with no progression Survival time (PFS) was statistically significantly improved.
Goxatuzumab is currently approved for the second-line treatment of metastatic triple-negative breast cancer in many countries around the world.
In May 2021, CDE accepted the marketing application of goxatuzumab for the treatment of triple-negative breast cancer and was included in priority review.
NO.2: The MAINTAIN study, a randomized phase II clinical trial:
evaluating the progression of antiestrogen therapy combined with CDK4/6 inhibitors, fulvestrant or exemestane with or without ribociclib ( Ribociclib) for the treatment of HR+/HER2- metastatic breast cancer patients
Speaker: Kevin Kalinsky Abstract number: LBA1004
Why it’s on the list: This is the first high-quality data on continued use of a CDK4/6 inhibitor after progression on a CDK4/6 inhibitor. What does it add to previous trials?
Med Supplementary Information:
At present, there is no high-quality evidence-based data on the regimen after CDK4/6 inhibitor treatment failure.
The exploration of clinical research can be roughly divided into 4 categories: 1. Continue to use CDK4/6 inhibitors and replace endocrine drugs 2.
To maintain endocrine therapy, replace another CDK4/6 inhibitor or other targeted drug; 3. To maintain CDK4/6 inhibitor and endocrine therapy, and add another targeted drug of the bypass pathway; 4. Other new drugs Clinical Trials.
NO.3: LUMINA study:
A prospective trial of deradiation after breast conserving surgery (BCS) for T1N0 luminal A breast cancer
Speaker: Timothy Whelan Abstract number: LBA501
Why it’s on the list: Could this change the standard of care for T1N0 luminal breast cancer?
Med Supplementary Information:
Adjuvant radiation therapy (RT) is currently the standard regimen after breast-conserving surgery in patients with early-stage invasive breast cancer.
But some patients have a lower risk of recurrence, and is there a way to screen them out to exempt them from radiotherapy?
During the 2021 ASCO meeting, abstract 512 further stratified the patient population of the SweBCG91 study and screened a signature containing 16 genes, named POLAR, suggesting that POLAR low-risk patients have no obvious benefit from radiotherapy, and more clinical trials are expected. Emergence of research data.
NO.4: HER3 ADC – Phase I/II study results of Patritumab deruxtecan in patients with HER3-expressing metastatic breast cancer
Speaker: lan Krop Abstract number: 1002
Why it’s on the list: ADCs are too hot right now, the story of HER3 ADCs may be similar to HER2 ADCs (T-DXd), should we be cooler?
Med supplementary information:
High expression of HER3 is closely related to tumor occurrence, development and prognosis of patients. Taking lung cancer as an example, in vitro studies have shown that the expression level of HER3 is related to tumor cell migration rate.
Patritumab deruxtecan (HER3-DXd, U3-1402) is currently the only HER3-ADC that has entered Phase II clinical trials. On December 23, 2021, Daiichi Sankyo announced that the FDA granted breakthrough therapy designation to patritumab deruxtecan for metastatic or locally advanced EGFR-positive non-small cell lung cancer (NSCLC) whose disease has progressed after receiving third-generation EGFR-TKIs and platinum-based drugs. )patient.
NO.5: Exploratory Analysis of KEYNOTE-522: Event-Free Survival (EFS) Outcomes of Pembrolizumab + Chemotherapy vs Placebo + Chemotherapy in Neoadjuvant Treatment of Early Triple-Negative Breast Cancer
Speaker: Lajos Pusztai Abstract number: 503
Why it’s on the list: Pathological complete response (pCR) may not be enough to assess prognosis, and residual tumor burden (RCB) may be a better indicator of risk.
Med Supplementary Information:
The primary endpoints of the KEYNOTE-522 study were pCR and EFS.
The first interim analysis showed that in patients with early-stage TNBC, pCR was significantly higher in patients receiving pembrolizumab plus neoadjuvant chemotherapy than in patients receiving placebo plus neoadjuvant chemotherapy.
In February of this year, the “New England Journal of Medicine” NEJM published the EFS results of the KEYNOTE-522 study. The previous pathological complete remission turned into a survival benefit, and this benefit was independent of PD-L1 expression levels.
However, one study alone cannot confirm that pCR can be used as a biomarker for early TNBC prognosis, especially a meta-analysis published in BMJ in 2021 showed that it cannot replace disease-free survival and overall survival.
Therefore, the authors of KEYNOTE-522 stated in the previous article that research on molecular biomarkers is still ongoing.
NO.6: Analysis of PALOMA-2 study: overall survival (OS) of palbociclib + letrozole versus placebo + letrozole in first-line treatment of women with ER+/HER2- advanced breast cancer
Speaker: Richard Finn Abstract number: LBA1003
Why it’s on the list: OS data for first-line palbociclib therapy have been missing in the past.
Med Supplementary Information:
Currently palbociclib has only obtained OS data in the phase II clinical trial PALOMA-1 study, which was prolonged but not statistically significant.
The median OS for palbociclib + letrozole vs letrozole was 37.5 vs 33.3 months (HR=0.813, 95%Cl 0.492-1.345, P[two-sided]=0.42).
In the past, PALOMA-2 only published the PFS results, so I am looking forward to the final OS results. I wonder if palbociclib can further verify its effectiveness.
NO.7: FAKTION study analysis:
Add OS data and update PFS data for biomarker stratified analysis. The FAKTION study was designed to evaluate fulvestrant + Capivasertib versus fulvestrant + placebo in patients with ER+ advanced breast cancer after progression on aromatase inhibitor therapy
Speaker: Robert Jones Abstract number: 1005
Reason for listing: The efficacy of single-agent fulvestrant is not satisfactory. In patients with ER+ advanced breast cancer, I wonder if it can help Capivasertib improve the efficacy?
Med supplementary information:
capivasertib is the first AKT inhibitor to enter clinical phase III development in the world, and its indications are mainly concentrated in HR+ breast cancer, triple negative breast cancer and prostate cancer.
Capivasertib has previously achieved positive results in the Phase II clinical trial PAKT study in patients with metastatic triple-negative breast cancer.
Capivasertib combined with paclitaxel in the first-line treatment of triple-negative breast cancer significantly prolonged the PFS of patients.
In 2020, AstraZeneca disclosed preliminary results from the FAKTION study.
Results of the study showed that patients randomized to fulvestrant plus capivasertib (n=69) had significantly longer mPFS (10.3 vs 4.8 m) and decreased disease progression compared to fulvestrant plus placebo (n=71) The risk was 42% (HR=0·58, 95%CI 0.39–0.84), meeting the primary endpoint of the study.
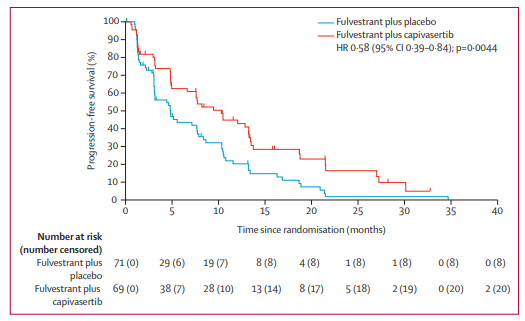
NO.8: Randomized phase III DESTINY-Breast03 study:
Safety follow-up of T-DXd versus T-DM1 in patients with HER2+ unresectable and/or metastatic breast cancer
Speaker: Erika P. Hamilton Abstract Number: 1000
Why it’s on the list: T-DXd is a 2nd line in the current standard of care. If we move it forward, how is the tolerability and safety performance of T-DXd?
Med Supplementary Information:
Previous safety results from the DESTINY-Breast03 study showed that the incidence of drug-related adverse events of any grade in the T-DXd and T-DM1 groups was 98.1% and 86.6%, respectively, and grade 3 or 4 drug-related adverse events. The incidence rates were 45.1% and 39.8%, respectively.
Drug-related interstitial lung disease or noninfectious pneumonia occurred in 10.5% and 1.9% of patients in the T-DXd and T-DM1 groups, respectively, but no grade 4-5 drug-related interstitial lung disease or noninfectious disease occurred. pneumonia.
NO.9: In the MONALEESA-2 study, the effect of Ribociclib dose adjustment on OS outcomes in HR+/HER2- advanced breast cancer
Speaker: Lowell Hart Abstract number: 1017 Poster number: 395.
Why it’s on the list: Did the dose reduction affect the key outcome data? I guess “no”.
Med supplementary information:
In March this year, NEJM officially announced the OS data of the MONALEESA-2 study after nearly 7 years of follow-up.
The median OS of ribociclib + letrozole vs placebo + letrozole was 63.9 vs 51.4 months (HR =0.76; 95%CI 0.63-0.93; two-sided P=0.008)
NO.10: NeoSTAR study results:
Phase II study of neoadjuvant therapy of goxatuzumab in local triple-negative breast cancer
Speaker: Laura Spring Abstract No.: 512 Poster No.: 284
Reason for listing: The exploration of ADC to advance to the stage of neoadjuvant therapy.
Med Supplementary Information:
The ASCENT study of goxatuzumab confirmed its efficacy in metastatic TNBC, and it is approved for second-line treatment of metastatic TNBC in several countries around the world. At present, goxatuzumab has also been actively explored in the first-line treatment of TNBC, as well as in the neoadjuvant and adjuvant treatment of early TNBC (NeoSTAR study and SASCIA study).
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



