New ADC Reduce risks of advanced breast cancer disease progression by 50%
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New ADC Reduce risks of advanced breast cancer disease progression by 50%
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New ADC Reduce risks of advanced breast cancer disease progression by 50%.
Reduce the risks of advanced breast cancer disease progression by 50%! The latest results of the blockbuster ADC are released.
On June 06, AstraZeneca and Daiichi Sankyo presented the blockbuster antibody-drug conjugate (ADC) Enhertu in the treatment of unresectable or metastatic patients with low HER2 expression at the plenary session of the ASCO annual meeting. Phase 3 clinical trial results in breast cancer patients.
The results showed that Enhertu reduced the risk of disease progression or death by 50% compared to chemotherapy in a population of patients with low HER2 expression!
The results mean that Enhertu has the potential to change the treatment paradigm for about half of breast cancer patients, the release notes.
In the 2020 global cancer statistics, breast cancer surpassed lung cancer as the most common cancer type worldwide.
Generally, breast cancer is divided into three types according to the type of receptor expressed by tumor cells, namely HR-positive/HER2-negative, HER2-positive, and triple-negative breast cancer.
The treatments for them vary by category. For breast cancer patients with HER2-positive tumors, targeted therapies that target HER2 are available. HER2-negative patients were previously ineligible for HER2-targeted therapy.
In this clinical trial, the Enhertu-treated patient population is referred to as HER2-low expression patients.
These patients are HER2-negative by traditional classification, yet their tumors actually express small amounts of HER2 and score 1+ on immunohistochemistry (IHC) staining, or 2+ on IHC but in situ hybridization (ISH) is negative.
The number of these patients is huge, accounting for up to 50% of breast cancer patients.
However, according to the current classification, they are not suitable for HER2-targeted therapy.
Previous monoclonal antibody therapies targeting HER2 have also not shown significant efficacy in this population.
Enhertu is an innovative antibody-drug conjugate jointly developed by AstraZeneca and Daiichi Sankyo.
In contrast to HER2-targeting antibody therapy, Enhertu does not need to inhibit HER2 activity to produce efficacy, the HER2 expressed on tumor cells is only a signal to guide the ADC to deliver cytotoxic drugs to the interior of the cell, which makes it possible to express lower levels of HER2. effect in tumors.
This innovative ADC uses Daiichi Sankyo’s technology platform called DXd ADC, and has made various technological innovations in ADC cytotoxic drugs and linkers.
For example, the DNA topoisomerase I inhibitor (DXd) conjugated with an antibody has a unique mechanism of action, and its activity is 10 times higher than that of the common chemotherapy drug irinotecan.
Moreover, this cytotoxic payload has a strong ability to penetrate the cell membrane, allowing them to kill nearby cancer cells after killing the cancer cells that have swallowed the ADC, resulting in a “bystander effect”.
Results from the Phase 3 clinical trial, DESTINY-Breast04, showed that Enhertu reduced the risk of disease progression or death by 49% (HR=0.51) compared with physician-chosen chemotherapy in a population of HR-positive, HER2-low-expressing patients , p<0.001). Median progression-free survival (PFS) was 10.1 months in the Enhertu group and 5.4 months in the chemotherapy group.
Notably, Enhertu reduced the risk of disease progression or death by 50% compared with chemotherapy in a population of patients with low overall HER2 expression regardless of HR positive or negative (HR=0.50, p<0.001). This patient population includes a subset of patients previously defined as triple-negative breast cancer.
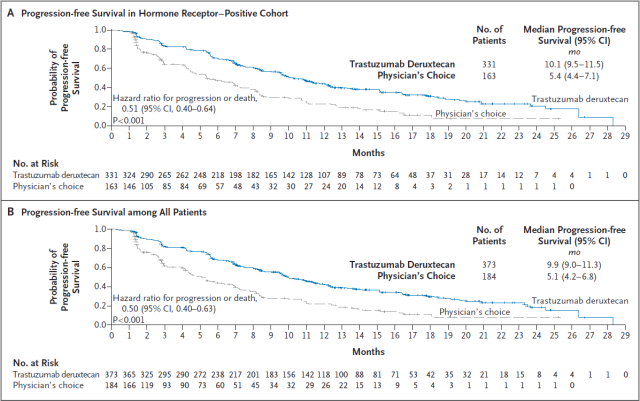
▲Enhertu positive HR, and PFS data in the entire patient population (Image source: Reference [3])
Enhertu has also shown excellent efficacy in prolonging the lives of patients. Reduced the risk of death by 36% compared with chemotherapy in the HR-positive patient population (HR=0.64, p=0.003), with a median overall survival (OS) of 23.9 months in the Enhertu arm and 17.5 months in the chemotherapy arm .
Enhertu also reduced the risk of death by 36% (HR=0.64, p=0.001) in the overall patient population with low HER2 expression, with a median OS of 23.4 months in the Enhertu group and 16.8 months in the chemotherapy group.
Detailed data have been published in the prestigious medical journal The New England Journal of Medicine.
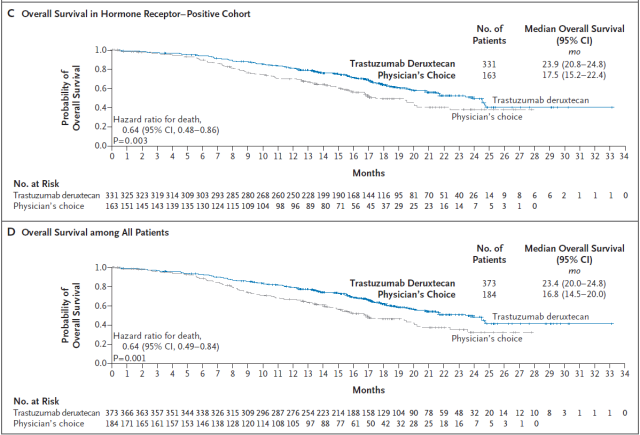
▲Enhertu is HR positive, and OS data in the entire patient population (Image source: Reference [3])
Exploratory analyses of different patient subgroups showed that Enhertu showed consistent efficacy regardless of whether the patients had been treated with CDK4/6 inhibitors, or the amount of chemotherapy they had previously received.
In the IHC 1+ patient population (meaning that more than 10% of cells were weakly or partially stained by immunohistochemistry), the efficacy of Enhertu was similar to that in the IHC 2+/ISH- patient population. In Asian patients, Enhertu showed a trend toward better efficacy.
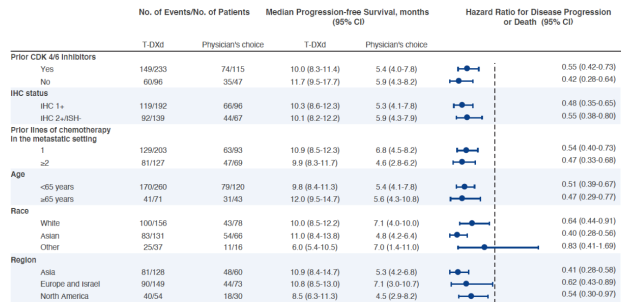
▲Analysis of subgroup efficacy data of Enhertu (Image source: Reference [3], click to enlarge)
Based on this positive result, the FDA has granted Enhertu Breakthrough Therapy Designation for this patient population.
“The DESTINY-Breast04 results are the first to show that HER2-targeted therapy can provide a survival benefit in patients with low HER2 expression,” said Shanu Modi, Ph. We must reconsider the way we classify patients with metastatic breast cancer.
The efficacy shown by Enhertu highlights its potential to provide a new standard of care for about half of patients with metastatic breast cancer!
These patients, although currently classified as HER2-negative, actually have tumors Express low levels of HER2.”
Appendix: List of DESTINY-Breast04 test results (Image source: Reference [1]
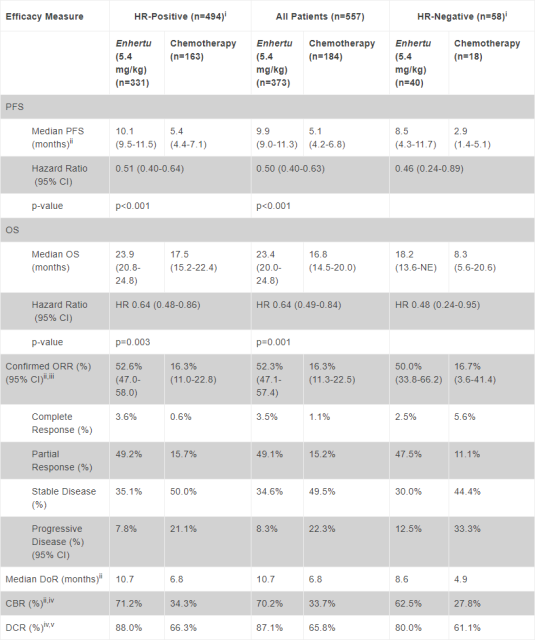
References:
[1] Enhertu reduced the risk of disease progression or death by 50% vs. chemotherapy in patients with HER2-low metastatic breast cancer with HR-positive and HR-negative disease. Retrieved June 5, 2022, from https://www. astrazeneca.com/content/astraz/media-centre/press-releases/2022/enhertu-efficacy-results-in-her2-low-breast-cancer.html
[2] Novel Antibody-Drug Conjugate Doubles Progression-Free Survival in Metastatic Breast Cancer With Low HER2 Expression Levels. Retrieved June 5, 2022, from https://www.asco.org/about-asco/press-center/news- releases/novel-antibody-drug-conjugate-doubles-progression-free
[3] Jacot et al., (2022). Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. The New England Journal Of Medicine, DOI: 10.1056/NEJMoa2203690
New ADC Reduce risks of advanced breast cancer disease progression by 50%
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



