Advances and Innovations in Toxicology Research in Pharmaceutical Industry
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Advances and Innovations in Toxicology Research in Pharmaceutical Industry
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Advances and Innovations in Toxicology Research in Pharmaceutical Industry
For decades, preclinical toxicology has been a largely descriptive discipline in which treatment-related influencing factors are analyzed in detail and used as the basis for calculating clinically safe dose ranges for drug candidates.
In recent years, however, technological advances have increasingly enabled researchers to gain insight into mechanisms of toxicity, continually improving new tools and strategies in toxicology research to reduce safety-related attrition in drug development.
Crucially, the goal of toxicology in the discovery phase is not simply ” preload ” depletion, but to increase the likelihood of a drug’s clinical success by optimizing the safety dimension of drug design and selection.
The key to successful drug toxicology studies is the effective integration of safety data with other specific molecular features, such as absorption, distribution, metabolism and excretion ( ADME ) and physicochemical properties, to address the inherent risks of potential targets and potential lead drugs.
This involves a paradigm shift away from the use of classical in vivo toxicology approaches towards translatable mechanistic in vitro assays that can serve as reliable predictive surrogates for in vivo studies.
In recent years, significant innovative advances have been made in areas such as induced pluripotent stem cells ( iPSCs ), 3D tissue models, microphysical systems ( MPS ), and imaging techniques that have the potential to greatly increase the predictive value of assays in toxicology studies.
Objectives and Methods of Toxicology Research
Toxicology studies can be broadly classified into two approaches: predicting potential toxicity before a compound undergoes nonclinical in vivo or clinical testing ( prospective approach ), or providing mechanistic understanding of nonclinical in vivo and clinical toxicity outcomes ( retrospective approach ).
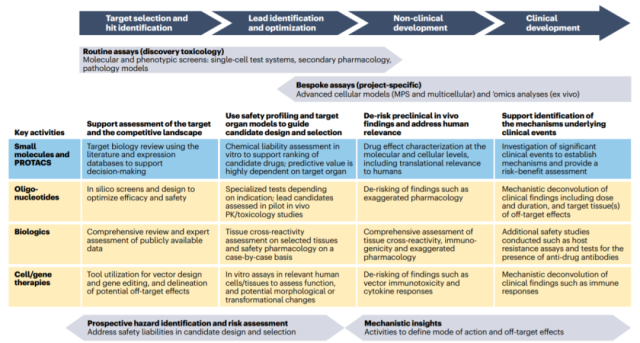
These approaches have two main goals: at the drug discovery stage, the aim is to guide the identification of the most promising drug candidates ( i.e., the safe candidates that offer the best therapeutic index ), and to exclude the most toxic candidates as early as possible.
During the preclinical and clinical development phases, the aim is to provide mechanism safety data that will enable sound risk assessment and management to support clinical trial design.
Evaluation of the target
In traditional toxicological assessment methods, some toxicological findings may be attributed to effects mediated by the drug’s primary target.
Over the past decade, traditional toxicological analysis has expanded to consider the broader physiological roles of targets in health and disease.
For example, the primary function of the target and the upstream and downstream signaling pathways, related targets ( orthologs and paralogs ), cross-species target homology analysis, functional and tissue expression across species, and expression of target genetic modifications in animals. type consequences.
A thorough target safety assessment facilitates the selection of the most appropriate species for preclinical safety studies. It can also identify potentially affected target organs and tissues for inclusion in the study, as well as biomarkers for monitoring the onset and extent of pharmacodynamic response.
Ultimately, the target safety assessment may inform the decision to continue or discontinue the study, as well as the experimental plan to quantify the risks as the project progresses further.
For example, the mammalian aspartic protease family is an important drug target. However, insufficient selectivity for certain aspartic proteases may hinder the development of safe and effective treatments.
Of these, cathepsins D and E are probably the most toxicologically relevant. Human cathepsin D deficiency has been reported to be the cause of congenital human neuronal ceroid lipofuscinosis characterized by neurodegeneration, developmental regression, vision loss, seizures, and premature degeneration due to excessive tissue accumulation of lipofuscin die.
Off-target inhibition of cathepsin D is a major driver of ocular toxicity for this class of drugs.
This example illustrates the need to thoroughly assess the risk of inhibiting closely related targets when developing drugs for a family of targets such as aspartic proteases.
Use in vitro models
Using in vitro cell models, organ-specific drug toxicity can be predicted and / or understood with varying levels of confidence .
However, since in vitro cytotoxicity and altered organ function are not necessarily linked, more sophisticated models have been developed to assess compound-induced perturbations in cellular function, thereby providing potential information on specific tissue and organ adverse effects.
Initially, people focused on measuring simple endpoints, such as cell viability by ATP content or other cytotoxicity endpoints in organ-derived cell lines, which are amenable to high-throughput screening (HTS) and are still heavily used in industry .
Recently, 2D and 3D cultures and microphysical systems have been developed in an attempt to mimic microenvironmental conditions, such as cardiac muscle derived from iPSCs or expanded pluripotent stem cells (ePSCs), by combining multicellular tissues, scaffolds, and cell-based mechanistic factors . cell.

Virtually every in vitro target organ approach, from simple cell lines to MPS, has inherent challenges and opportunities, so the use of multiple “fit-for-purpose” assays best represents current practice.
Simple cell cultures with relatively straightforward endpoints can provide high-throughput and robust methods for screening and studies of specific mechanisms, but do not require the complexity required to reflect the in vivo situation; while the complexity can be complemented by organoids or MPS to Provides deep mechanistic understanding.
Preclinical studies elucidate relevance to humans
In vitro assays may help to understand the molecular and cellular mechanisms underlying pathological effects observed in various animal studies and to elucidate their potential relevance to humans.
The following example illustrates the impact of understanding the mechanism of action of toxicity as a drug candidate enters clinical trials or enters the market.
Drug-induced tumor changes are a common occurrence in in vivo safety studies, especially in rodents.
For example, altered hepatocellular foci are considered possible preneoplastic lesions that can occur spontaneously or be induced by chemicals or drugs. But in carcinogenicity studies, increased lesions in rodents did not necessarily lead to tumors.
The non-genotoxicity of RG3487, a partial nicotinic α7 receptor agonist candidate, has been found to induce hepatocyte changes in rats, which in turn induce tumors, which may hamper human clinical studies.
However, the changes in hepatocytes seen in rats were not seen in mouse or dog livers.
To assess human relevance, rodent, canine, and human stem cells, as well as phenotyping of patient-derived 3D cell models, were used to explore potential mechanisms of tumorigenesis.
Rodent phenotype models were found to exhibit in vivo effects, whereas human and canine models apparently showed no effects, and studies showed drug-induced effects of nuclear receptor-driven liver proliferation in rodents but not in human models.
These data support the entry of RG3487 into clinical trials. The data from this case highlight the importance of using cross-species in vitro models for human toxicology studies at the molecular and phenotypic levels.
Identify underlying mechanisms of clinical events
Toxicology studies are also critical when assessing safety concerns arising after clinical development or approval.
This support is not limited to the parent drug, but may also include drug impurities, degradants, and metabolites.
For example, in anemic patients treated with recombinant human erythropoietin ( rhEPO ), immunogenic consequences arising from aggregated forms of rhEPO may lead to pure red cell aplasia ( PRCA ), so understanding the underlying cause of protein aggregation is critical for safe use rhEPO is crucial.
T-cell assays confirmed the immunogenicity of tungsten-induced rhEPO aggregates in a clinical batch associated with a case of antibody-mediated PRCA, a finding that facilitated regulatory approval as part of a root-cause analysis.
Therefore, the product ended up modifying the production mode of the syringe to avoid the possibility of tungsten contamination.
Post-mortem investigations of adverse events in clinical trials do not always save the product, but may provide solutions for subsequent drug candidates.
For example, after an anti-CD40L antibody was terminated from clinical development for the treatment of autoimmune diseases due to unexpected thrombotic complications, studies using a vessel-on-a-chip model revealed a mechanism for prothrombotic effects.
Modification of subsequent antibodies with an Fc domain that does not bind to the FcγRIIa receptor on human platelets attenuates the prothrombotic effect.
Major Innovations in Toxicology Research

Imaging technology
Spatial resolution of drug effects on cells and tissues provides valuable insights into drug toxicity; moreover, multiparametric imaging of biomarkers provides the basis for mechanistic understanding.
Significant advances and applications have been made in in vitro and in vivo imaging techniques, including high-content imaging ( HCI ), fluorescence and bioluminescence imaging, MRI, CT, and high-dimensional molecular profiling of cell and tissue samples using MSI.
Omics technology
Toxicogenomics ( TGx ), the molecular assessment of toxicological effects through transcriptomics or the cellular output of gene expression ( i.e., proteomics and metabolomics ). These techniques can greatly enhance preclinical toxicology studies.
Transcript profiling to prioritize compounds during early drug discovery is a common approach in the pharmaceutical industry.
Gene expression profiles of compounds can be used to capture a range of polypharmacological effects and can be used to monitor the impact of medicinal chemistry optimization.
In addition, it can be used to speculatively analyze what compound substructure is responsible for a given effect, and ultimately support medicinal chemists in designing new synthetic structures.
Recently, transcriptome-based and drug-induced genomic effect analysis approaches have been shown to explain the mechanism of liver toxicity and predict distinct toxicity phenotypes using gene network module associations.
Computation and Modeling to Predict Drug Toxicity
The integration of multiple test benches generates large datasets that require the use of high-performance computing and machine learning-based modeling to adequately analyze mechanisms or make effective predictions.
Models based on in vitro assay data may perform better than animal toxicity studies in predicting human toxicity in certain specific circumstances.
A prime example of such an approach is predicting myelosuppression of oncology drugs, which in the past has often been a dose-limiting toxicity.
To predict and better inform the time course of myelosuppression following drug administration, semiphysiological mathematical models have been constructed.
These models can predict not only the degree of myelosuppression induced by novel drugs, but also how they might interact with myelosuppressive standard-of-care therapies.
This enables the most favorable oncology drug candidates to be prioritized and can also be explored through combination dosing studies to guide clinical use.
iPSCs and Advanced Cell Models
To generate more translationally relevant data, toxicologists are increasingly using complex human and animal MPS models to gain insight into organ-specific and inter-organ toxicity profiles.
iPSC-derived cardiomyocytes ( iPSC CMs ) are the most advanced iPSC-derived models used in the pharmaceutical industry to assess cardiotoxicity and cardiac electrophysiology.
Advances in 3D hepatocyte culture systems have led to further improvements in function at the physiological tissue and organ level.
Furthermore, hepatic MPS based on primary hepatocytes as well as nonparenchymal and immune cells has the potential to mimic the genetic, physiological, and disease environment of the donor and replicate the toxicology of specific environments.
In addition, substantial progress has been made in other MPS tissue models, including the gastrointestinal tract, lung, and kidney.
These models still need to be developed and validated to be suitable for drug discovery and safety testing.
Gene editing
The development of gene editing platforms such as CRISPR–Cas9 has allowed researchers to gain insight into the contribution of single gene products to disease.
The ability to accurately and specifically delete or modify specific genes can also provide insight into the potential safety of drug targets when focusing on understanding specific biological mechanisms of toxicity or pathways of adverse outcome.
Summary
Drug toxicology is based on the physical and chemical properties of drugs , using the principles and methods of toxicology , to conduct a comprehensive and systematic safety evaluation of drugs and to clarify their toxic mechanisms.
Its main purpose is to guide clinical rational drug use , reduce adverse drug reactions and reduce new drug development failures caused by drug toxicity.
In recent years , with the rapid development of various cutting-edge disciplines and related technologies , drug toxicology has been given a new opportunity for development.
Especially in recent years, the research model of drug toxicology is also undergoing tremendous changes to meet the actual needs of early decision-making in drug development, shortening the time and cost of new drug development, and in the process of research on toxicity issues that are most likely to lead to the failure of new drug development Facilitate close collaboration among pharmaceutical industry, academia and regulatory authorities.
references:
1. The evolving role of investigative toxicity in the pharmaceutical industry. Nat Rev Drug Discov. 2023 Feb 13;1-19.
Advances and Innovations in Toxicology Research in Pharmaceutical Industry
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



