NEJM: New Breakthrough in Elderly Refractory Depression!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
NEJM: New Breakthrough in Elderly Refractory Depression!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NEJM: New Breakthrough in Elderly Refractory Depression! Scientists find that adding antipsychotic drug aripiprazole is effective in remission
Relevant data show that about 4.3% of people in the world suffer from depression, and the three groups with the highest risk are young people, pregnant women, and the elderly [1].
In the elderly population, depression is the disease with the highest disability rate , which is manifested in the damage of brain function and the decline of quality of life. Depression is also the most common symptom of Alzheimer’s disease [2].
Elderly depression not only affects patients’ daily sleep, but also seriously affects patients’ daily life, especially refractory elderly depression. If the diagnosis and treatment are not timely, it is easy to induce psychosomatic diseases.
Studies have shown that 20%-30% of patients with depressive disorders are ineffective or ineffective after antidepressant drug treatment , which belongs to treatment-resistant depression [3]. For treatment-resistant depression, the current commonly used strategies are mainly to switch to other different antidepressant drugs , or to add other types of drugs . However, which therapy is more effective remains to be further studied.
Recently, the team of Eric J. Lenze, a professor of psychiatry at Washington University in St. Louis , published the latest research results in the top medical journal New England Journal of Medicine [4].
They found that adding aripiprazole, an antipsychotic mainly used to treat schizophrenia , was more effective than switching to another antidepressant in the treatment of treatment-resistant older adults, leading to a significant improvement in Patient well-being, eased incidence of depression .
Screenshot of paper home page
The 2-year, 10-month, two-step, open-label trial enrolled 742 patients over the age of 60 with treatment-resistant depression, all of whom had nine Patient Health Questionnaire (PHQ-9) scores of 10 or greater (0-27 points, the higher the score, the more severe the depressive symptoms) .
A total of 619 patients with treatment-resistant depression participated in the first step trial, with an average age of 69.3 years, and women accounted for 66.7%.
In the first step trial, patients were randomly assigned to either augment with aripiprazole (n=211) or bupropion (n=206) or to switch from their current drug to bupropion (n=202) .
Patients whose condition did not remission in the first step trial or who did not meet the treatment conditions of the first step participated in the second step trial.
A total of 248 patients were enrolled in the second step trial, with an average age of 68.5 years and 69.8% of them were female. Patients were randomly assigned to lithium enhancement (n=127) or switching from current medication to Nortrilen (nortriptyline) (n=121) .
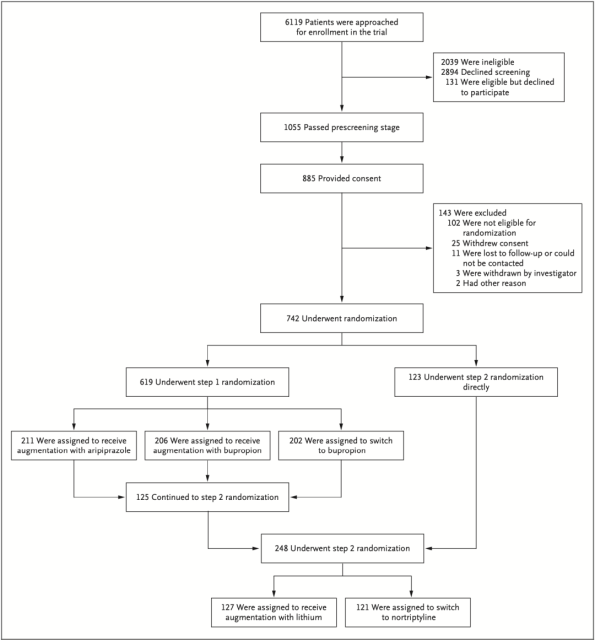
Grouping and patient characteristics of the two-step trial
Lenze et al. first assessed the effectiveness of different treatment options in elderly patients with treatment-resistant depression.
The primary efficacy outcome was mental health as quantified by the National Institutes of Health Positive Affect and General Life Satisfaction subscale (higher scores correspond to higher well-being) , and the secondary efficacy outcome was quantified by the MADRS Depression Scale Depression, social participation, and changes in physical function of patients.
In the first step trial, patients in the aripiprazole-boost group had a 4.83-point increase in mental health scores from baseline, and those in the bupropion-boost group had a 4.33-point increase in mental health scores after switching from current medication to amphetamine Patients on keto had a 2.04 point increase in mental health scores.
The mental health score of the enhanced aripiprazole group increased by 2.79 points more than that of the group switched to bupropion (P=0.014, with a significant difference). The increased difference was 2.29 points (P=0.049, not significant difference) .
These results suggest that augmentation with aripiprazole is more effective than switching from the current drug to bupropion in the treatment of elderly patients with treatment-resistant depression.
In the second step trial, patients who received lithium-augmented therapy increased their mental health scores by 3.17 points from baseline, compared with a non-significant increase of 2.18 points in patients who switched to nortriptyline.
In terms of remission of depression, 28.9% of patients in the aripiprazole augmentation group had remission of depression compared to those in the bupropion group (19.3% remission of depression and 4.14 reduction in MADRS score) ( HR=1.50) , the MADRS score decreased by 7.60 ; the percentage of patients in the bupropion augmentation group who experienced depression remission was 28.2% (HR=1.49) , and the MADRS score decreased by 7.23 .
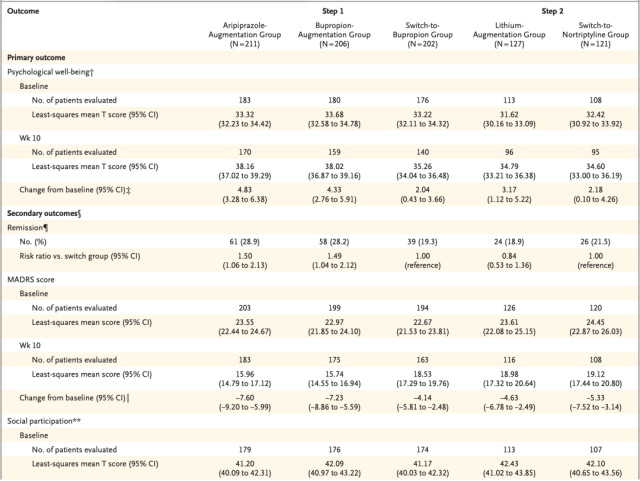
Effectiveness of different treatment options for elderly patients with treatment-resistant depression
In the second-step trial, the percentage of patients in the lithium-enhanced group who were relieved of depression was 18.9%, and the MADRS score was reduced by 4.63; the percentage of patients who were switched to nortriptyline was 21.5% (HR=0.84), and the MADRS score was reduced by 21.5 % . 5.33.
The above experimental results show that, compared with switching from the current drug to bupropion, aripiprazole enhancement and bupropion enhancement are more effective for treatment-resistant depression in the elderly, and have a better effect on alleviating depression .
That is to say, for the treatment-resistant depression in the elderly, the treatment effect of “adding medicine” is better than “changing medicine”. Lithium augmentation and switching to nortriptyline were similar in efficacy for treatment-resistant depression in the elderly.
Next, Lenze et al. further evaluated the safety of different treatment options for elderly patients with treatment-resistant depression.
The main safety outcome was the evaluation of falls during the drug administration of patients. The main indicators included slipping or tripping, loss of balance, falling on the floor or ground or a lower level, and whether the fall resulted in injury.
In the first step trial, patients in the aripiprazole boost group had a fall rate of 0.33, those in the bupropion boost group had a fall rate of 0.55, and those in the bupropion switch group had a fall rate of 0.38 .
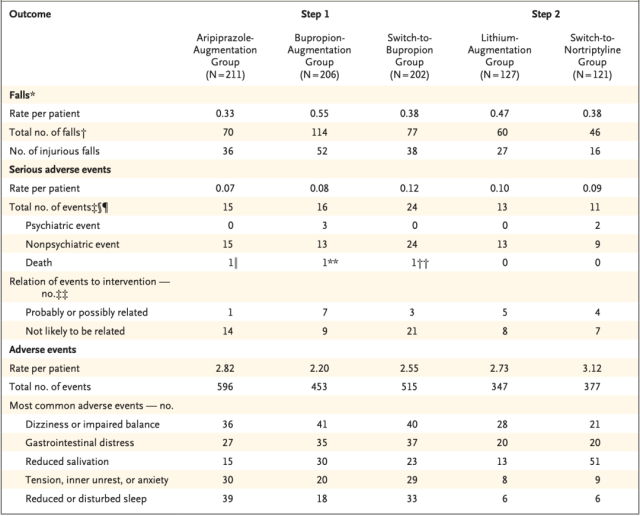
Safety of different treatment regimens in elderly patients with treatment-resistant depression
Specifically, the overall serious adverse event rate was 0.07 (HR=0.59) in the aripiprazole boost group compared with the switch to bupropion group (0.12 overall serious adverse event rate) , and the bupropion group had an overall serious adverse event rate of 0.07 (HR=0.59). The overall serious adverse event rate in the booster group was 0.08 ( HR= 0.61) .
In the second step trial, patients in the lithium augmentation group had a fall rate of 0.47 compared with 0.38 in the nortriptyline group (HR=1.22) , and serious adverse event rates were 0.10 and 0.09 (HR=1.30), respectively. ) .
Overall, the rates of adverse events and serious adverse events were similar among elderly patients with treatment-resistant depression who received “add-on” therapy and those who received “drug-switch. “
Of note, even in the group with the lowest risk of falls (aripiprazole boost) , a fall rate of 0.33 meant that 1 in 3 patients fell during approximately 10 weeks of treatment, so in the elderly refractory During the diagnosis and treatment of sexual depression, special attention should be paid to falls in the elderly.
In summary, this study shows that for elderly refractory depression, “additional drug” is more effective than “drug change”, and bupropion enhancement and aripiprazole enhancement are effective Effectiveness for sexual depression was numerically similar, both significantly higher than switching to bupropion, but bupropion augmentation was associated with higher rates of falls .
Therefore, in terms of comprehensive efficacy and safety, aripiprazole enhanced treatment may be a better overall antidepressant strategy for elderly patients with treatment-resistant depression.
references:
[1] DILLON DG, PIZZAGALLI D A. Mechanisms of memory disruption in depression[J].Trends Neurosci, 2018, 41(3): 137-149.
[2] Davide Cappon, Tim den Boer, Caleb Jordan, Wanting Yu, Eran Metzger, Alvaro Pascual-Leone, Transcranial Magnetic Stimulation (TMS) for Geriatric Depression, Aging Research Reviews, 2021, 101531, ISSN 1568-1637.
[3] Yang Shufen, Yang Lishen, Xia Yongbing, et al. Efficacy of aripiprazole combined with sertraline on patients with schizophrenia and depression. Chinese General Medicine, 2018, 21(2):231-232.
[4] Lenze EJ, Mulsant BH, Roose SP, et al. Antidepressant Augmentation versus Switch in Treatment-Resistant Geriatric Depression [published online ahead of print, 2023 Mar 3]. N Engl J Med. 2023;10.1056/NEJMoa2204462.
NEJM: New Breakthrough in Elderly Refractory Depression!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



