FDA urgently approved the import of this drug from China due to shortage
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA urgently approved the import of this drug from China due to shortage
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA urgently approved the import of this drug from China due to shortage. The US FDA is relaxing import rules for some chemotherapy drugs. [1]

Source: Reference 2
According to relevant media reports, the US FDA plans to import a cisplatin injection (Cisplatin Injection) from Qilu Pharmaceutical in China to alleviate the shortage of drugs in the US.
According to the information from the State Drug Administration , the drug was produced by Qilu Pharmaceutical Co., Ltd. and was approved on October 26, 2021. The approval number is “Guoyao Zhunzi H20213819”.
It is reported that this cisplatin injection has not been approved by the FDA before, and this is the first time that a Chinese pharmaceutical company has directly supplied the shortage drug to the United States with a product sold in the domestic market. [3]
FDA Announces Drug Shortages, Qilu Pharmaceuticals Among Demanders
Cisplatin injection is a first-line chemotherapy drug for the treatment of various tumors.
Judging from the information published on the FDA’s official website, five cisplatin pharmaceutical companies, including Accord Healthcare and Hikma Pharmaceuticals, have experienced shortages and delayed delivery of cisplatin injections to varying degrees, and this shortage will continue until 2023 May or the third and fourth quarters, and gradually recover thereafter. [2]
The drug has been in “short supply” since February 10 this year.
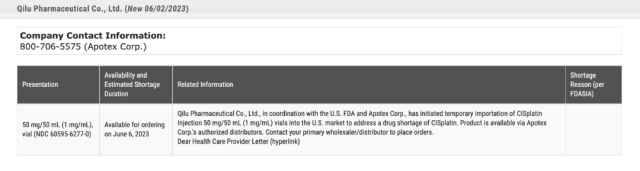
FDA Updates Qilu Pharmaceuticals Supply Situation
Source: Reference 2
According to Jinan Daily, in March this year, an email from the FDA suddenly arrived at the mailbox of Qilu Pharmaceutical, asking whether it could provide cisplatin injection, a drug in short supply in the US market.
Zhang Hanchang, vice president of Qilu Pharmaceutical Group and general manager of Qilu Pharmaceutical Co., Ltd., said in an interview with Jinan Daily that the company completed the preparation of materials, application of plans, and several rounds of communication and consultation with the FDA. Product delivered . [3]
Subsequently, Qilu Pharmaceutical stated in its reply letter to the FDA that due to the severe shortage of cisplatin for injection in the United States, Qilu Pharmaceutical Co., Ltd. will use Apotex as the distributor to jointly temporarily export cisplatin with Chinese carton labels to the US market under the coordination of the FDA. Bottled cisplatin injection (50mg/50mL).
In addition, Qilu Pharmaceutical reminded: Cisplatin injection is a prescription drug in the United States, but the label of the imported batch is not clearly marked as “Rx only”, and the label of relevant information has been pasted on the vial and carton; The linear barcode of the product may not be accurately registered on the scanning system in the United States, and drug information needs to be imported manually; the packaged carton does not have the product identifier required by the “Drug Supply Chain Security Act”, etc. [4]

Source: Reference 4
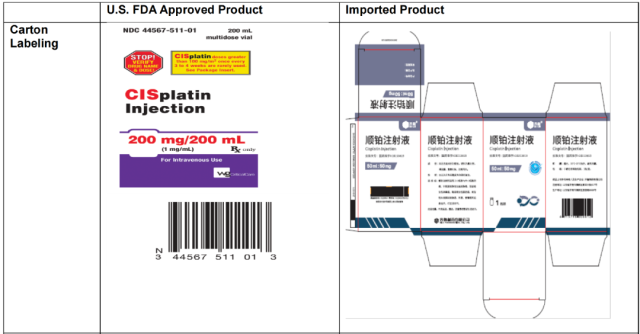
Product Label Comparison
Source: Reference 4
The shortage is not temporary, it is caused by limited production
In response to the reason for this shortage, Richard Pazdur, acting director of the FDA Office of Oncological Diseases, said in an exclusive interview that this is the final result of a “failure in the market”. [5]
According to Pazdur, the current shortage of cisplatin needs to be traced back to a manufacturing facility inspection — which resulted in cisplatin after an inspection of a pharmaceutical company’s manufacturing plant revealed quality issues that shut down production lines to fix the problem. current shortages.
Pazdur said that as prices and margins fall, manufacturers may weaken the supply chain elasticity, thus making the supply of drugs from a single source. Due to the chain reaction of the shortage of cisplatin, the market demand for carboplatin will increase, which will also face challenges.
As of May 2023, there will be shortages of 17 tumor-related drugs, including capecitabine tablets, carboplatin injection, and cisplatin injection.
Previously, in order to solve the plight of drug shortages, two medical experts at the University of Chicago signed an article A proposal for the Cancer Moonshot: Set up a strategic reserve for lifesaving generic cancer drugs [6], proposing to “establish an anti-cancer and life- saving generic drug Strategic Reserve” as part of the cancer moonshot program. Among them, cytarabine, carboplatin, cisplatin, cyclophosphamide, methotrexate, paclitaxel, etc. are included.
But so far, the proposal has not been approved. [5]
In a May 30 interview, Pazdur said that in addition to importing drugs, the FDA is also exploring whether it can extend the expiration date of existing drugs.
Compilation: Little Swallow | Planning: carollero | Producer: gyouza
Title picture source: Screenshot of Jinan Newspaper’s all-media video
References:
[1]. https://www.cnbc.com/2023/06/02/cancer-drug-shortage-fda-allows-import-of-unapproved-china-chemo-med.html
[2] . https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Cisplatin%20Injection&st=c&tab=tabs-1
[3]. h ttp://innovation.jinan.gov.cn/art/2023/6/2/art_16571_4803321.html
[4]. https://www.fda.gov/media/169001/download
[5]. https://cancerletter.com/conversation-with-the-cancer-letter/20230530_1/
[6]. https://cancerletter.com/guest-editorial/20230428_5/
FDA urgently approved the import of this drug from China due to shortage
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



