First release of base-edited CAR-T clinical data for children with T-ALL
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
- Aspirin Found Ineffective in Improving Recurrence Risk or Survival Rate of Breast Cancer Patients
- Child Products from Aliexpess and Temu Contain Carcinogens 3026x Over Limit
- Daiichi Sankyo/AstraZeneca’s Enhertu Shows Positive Results in Phase III DESTINY-Breast06 Clinical Trial
- Mn007 Molecules Offer Potential for Combating Streptococcus pyogenes Infection
- Popular Indian Spices Banned in Hong Kong Over Carcinogen Concerns
First release of base-edited CAR-T clinical data for children with T-ALL
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First release of base-edited CAR-T clinical data for children with T-ALL.
Base editors, the deamination of cytidine guided by clustered regularly interspaced short palindromic repeats (CRISPR), can mediate the conversion of one nucleotide into another with high precision—especially cytosolic pyrimidine (C) to thymine (T) without DNA breakage.
Thus, genes can be edited and inactivated by base editors without inducing translocations and other chromosomal aberrations. Currently, this technology is being used to explore the treatment of relapsed/refractory childhood acute T-cell leukemia (T-ALL) .
Recently, Professor Waseem Qasim from UCL Great Ormond Street Institute of Child Health and his clinical team published an article in The New England Journal of Medicine on Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia .
The interim results of this phase I clinical trial showed that 2 subjects achieved sustained remission after infusion of spot-type CD7 CAR-T (CD52, CD7 and TRBC were simultaneously inactivated) combined with allogeneic stem cell transplantation; the other 1 Subject died due to complications of fungal infection.
The results of this trial preliminarily support the continued use of base-edited spot CAR-T in children with T-ALL. The adverse side effects and potential risks of complications provide sufficient experience and treatment plans for subsequent clinical trial exploration.

Main method: In this clinical trial, spot-type CAR-T (BE-CAR7 T cells) is used , that is, T cells from healthy people use base editors to simultaneously inactivate CD52, CD7 and TRBC to avoid accidental killing of lymphoma, CAR-T cells cannibalism and GVHD events occur.
Then transduce the anti-CD7 CAR sequence into T cells to obtain BE-CAR7 T cells, which can be used to treat T-ALL in children. The specific principle is shown in Figure 1.
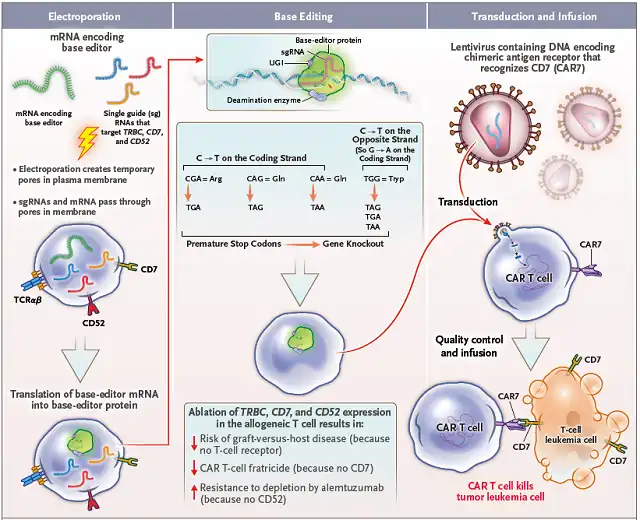 Figure 1 Schematic diagram of the preparation and working principle of BE-CAR7 T cells
Figure 1 Schematic diagram of the preparation and working principle of BE-CAR7 T cells
Treatment plan: The subject first needs to be treated with drenching, fludarabine: 150 mg/m 2 ; cyclophosphamide: 120 mg/kg; alemtuzumab (anti-CD52 antibody) : 1 mg/kg. 0.2-2.0×10 6 cells/kg of BE-CAR7 T cells were infused after detoxification treatment (TCRαβ+ T cells were not allowed to exceed 5×10 4 cells/kg to ensure that the risk of GVHD would not occur) .
After 28 days of infusion, if the disease achieves molecular remission (molecular remission) , non-myeloablative allogeneic stem cell transplantation will be adopted, and then the efficacy and safety of the drug will be followed for a long time.
Subject 1: girl, 12 years old, T-ALL (t(10;11) chromosome translocation and chromosome 9p deletion) , morphological disease remission after multiple lines of chemotherapy and MRD positive, no central nervous disease and extramedullary lesions, After receiving HLA-matched allogeneic stem cell transplantation, MRD was still positive after 4 weeks, and relapsed after 4 months, and all blast cells were CD7 positive.
Subsequently, the subject received detoxification and infusion of 50×10 6 BE-CAR7 T cells. The safety profile was good.
Grade 2 CRS and Grade 1 ICANS occurred, and no inflammation or GVHD was induced. BE-CAR7 T cells expanded well, achieved morphologic disease remission and were MRD negative 27 days after infusion.
Subsequently, non-myeloablative allogeneic stem cell transplantation was performed on the 43rd day, which caused adverse side effects such as grade 2 GVHD and mucositis, and was discharged on the 52nd day.
At the 9th month after the allogeneic stem cell transplantation, the sustained remission at the molecular level was still maintained, and the level of immunoglobulin and the number of each subset of lymphocytes were within the normal range, as shown in Figure 2.
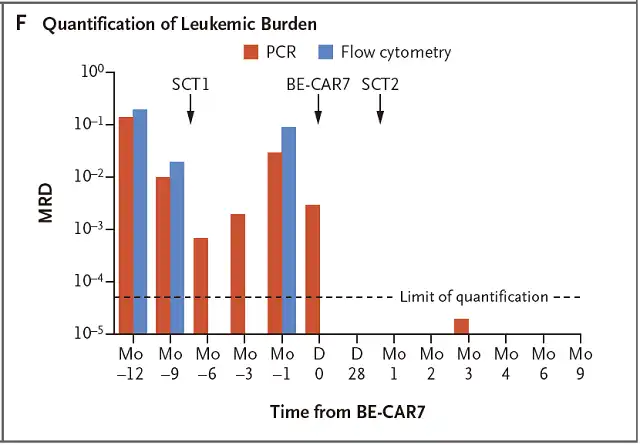 Figure 2 MRD detection of leukemia burden index in subject 1
Figure 2 MRD detection of leukemia burden index in subject 1
Subject 2: Boy, 13 years old, diagnosed with relapsed/refractory T-ALL, 80% of bone marrow are blast cells and all express CD7.
Then the subject received debridement and was infused with 1×10 6 BE-CAR7 T cells/kg, which caused more severe CRS, and fungal infection complications occurred at the same time, and died on the 33rd day.
Subject 3: A 15-year-old boy who was first diagnosed with mixed acute leukemia and relapsed after receiving successful allogeneic stem cell transplantation and multiple lymphocyte infusions.
Subsequently, he received allogeneic stem cell transplantation from umbilical cord blood, and relapsed again.
The original mixed acute leukemia transformed into CD7-positive T-ALL, accompanied by central nervous system disease. The subject was infused with 0.9×10 6 BE-CAR7 T cells/kg after detoxification , which resulted in grade 2 CRS and no ICANS occurred.
On the 28th day, the findings were confirmed as sustained remission at the morphological and molecular levels, followed by preparation for allogeneic stem cell transplantation.
In summary, the interim results of this phase I clinical trial initially support the continued use of base-edited spot-type CAR-T in children with T-ALL, and the adverse side effects and potential complications provide sufficient evidence for subsequent clinical trial exploration. experience and treatment plans .
This clinical trial was funded by the Medical Research Council (ISRCTN15323014) .
Original link:
First release of base-edited CAR-T clinical data for children with T-ALL
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.