The world first drug treatment of spinal cord injury
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world first drug treatment of spinal cord injury
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The world first drug treatment of spinal cord injury: Clinic data released, patient benefit trend.
Traumatic spinal cord injury has always lacked effective treatment methods in clinical practice . It is comparable to a “cancer” that does not kill people. The blow to patients themselves and their families is incalculable. If this problem can be solved, it will be comparable to the discovery of “normal temperature and pressure superconducting materials” in the medical field.
Stem cell transplantation[1], electrical stimulation of the spinal cord[2], and exoskeleton robots[3], all of which sound very grand, have all been tried to be used in the treatment of spinal cord injuries, but this is also from the side Reflecting the difficulties in the treatment of spinal cord injury. After all, if it can be solved by taking medicine, why bother to do it so hard.
The good news is that treating spinal cord injuries with drugs no longer seems out of reach .
Recently, the Stephen M Strittmatter team from Yale University School of Medicine published the results of the first clinical trial in the Lancet Neurology to treat chronic spinal cord injury by promoting nerve repair with drugs [4].
In this study, the researchers evaluated the safety and preliminary efficacy of AXER-204, a decoy drug containing multiple ligand-binding domains of NgR1 (a key pathway for axon regeneration), in patients with chronic cervical spinal cord injury (more than 1 year).
The results showed that the subjects could tolerate 200 mg of AXER-204 intrathecal injection treatment, and the average maximum cerebrospinal fluid concentration on the first day after administration was 412,000 ng/mL, exceeding the effective concentration in animal experiments . Throughout the study, no serious adverse events related to AXER-204 were noted.
In addition, the preliminary efficacy analysis showed that the change in the International Neurological Classification Standard Upper Extremity Motor Score (ISNCSCI UEMS) for spinal cord injury in patients receiving 200 mg intrathecal injection of AXER-204 on day 169 was superior to that in the placebo control group trend (1.5 vs 0.9, p=0.59), and may be more effective in patients with moderate to severe spinal cord injury who have not received AXER-204 treatment .

Screenshot of paper home page
Disconnection and axonal damage resulting from spinal cord injury often lead to persistent neurological dysfunction.
In theory, recovery of function could be promoted by promoting axonal regeneration . However, the weak repair ability of nerve cells and a variety of extracellular environmental factors that inhibit axon regeneration make this treatment strategy difficult[5].
Oligodendrocytes are one of the “culprits” that inhibit the microenvironment of axon regeneration. The Nogo, MAG and Omgp secreted by it can bind to the NgR1 receptor on the neuron membrane, thereby inhibiting axon growth[6].
AXER-204 is obtained by fusing the binding domains of NgR1 and the above three proteins to the Fc segment of IgG1 , and its therapeutic effect has been demonstrated in various rat central nervous system injury models [7] and non-human primates. Spinal cord injury research [8] was confirmed.
On this basis, the Strittmatter team launched a human clinical trial (NCT03989440) to evaluate the safety and pharmacokinetics of AXER-204 in patients with chronic spinal cord injury, and to initially explore the therapeutic effect.
Subjects included in the study were patients aged 18-65 with chronic (>1 year) traumatic cervical spinal cord injury.
The test was divided into two parts. In the first part, subjects received a single intrathecal injection of 3 mg, 30 mg, 90 mg or 200 mg of AXER-204 to evaluate the safety and pharmacokinetics of AXER-204.
The second part is a randomized (1:1), double-blind controlled trial, while further evaluating the safety and pharmacokinetics of AXER-204 (200mg/time, expected to be 6 times), comparing it with placebo Differences in treatment effects.
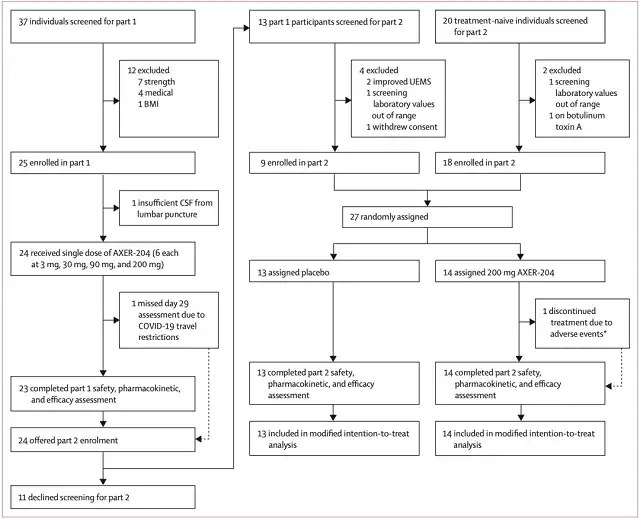
Research flow chart
A total of 24 patients were enrolled in the first part, 6 per dose, with a mean age of 38.8 years and a mean time since injury of 51.5 months.
A total of 27 patients were enrolled in the second part (9 patients from the first part), 14 in the AXER-204 group and 13 in the placebo group, with an average age of 38 years and a mean time since injury of 107.8 months.
In the second part, the vast majority of subjects received the projected 6 treatments, with an average of 5.7 in the AXER-204 group and 5.9 in the placebo group.
Headache was the most common adverse event (treatment-related or not) in either part I or part II, with 12 patients (50%) in part I and 19 patients (70%) in part II ) reported headaches . Most of these headaches were related to lumbar puncture, and there were no grade 3 or 4 headaches throughout the trial.
In the first part, no patients experienced serious adverse events . In the second part, 4 serious adverse events were reported in 4 patients (29%) receiving AXER-204 and 4 serious adverse events also occurred in 2 patients (15%) receiving placebo.
Urinary tract infection and pressure ulcers are common serious adverse events in patients with spinal cord injury.
However, most SAEs were considered unrelated to treatment, with only one treatment-related SAE (constipation) reported in the placebo group. No patient withdrew from the study due to adverse events .
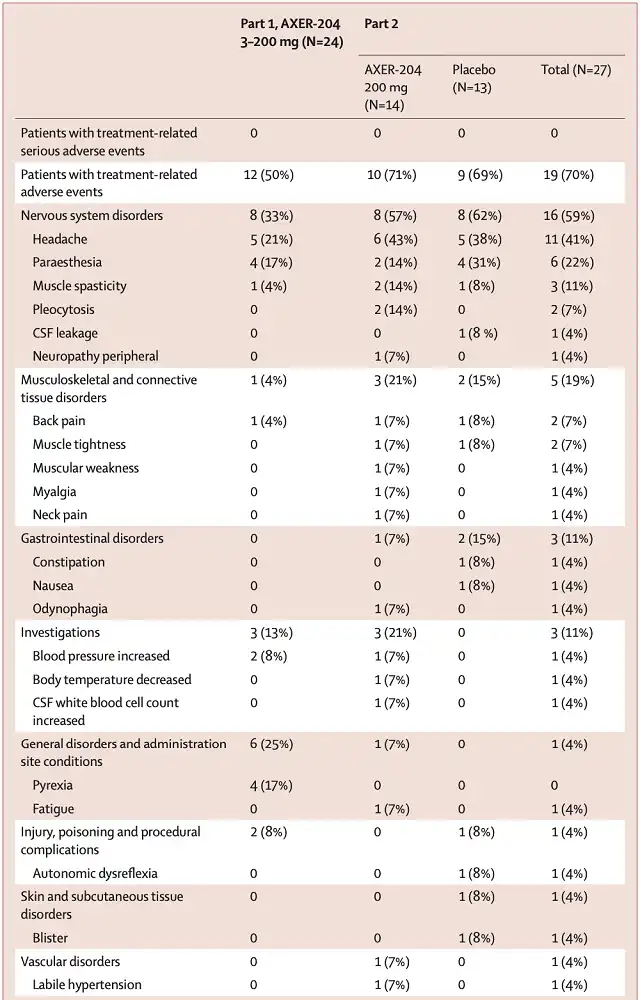
Occurrence of adverse events
For pharmacokinetics, in the first part of the test, the maximum cerebrospinal fluid concentration in the 200 mg group was 412,000 ng/mL 24 hours after administration, and the average half-life was 12.5 hours.
Serum AXER-204 concentrations reflecting systemic exposure were not quantifiable at the 3 mg and 30 mg doses, with a mean peak concentration of 641 ng/mL in the 200 mg group.
In the first part of the trial, results of CSF and serum concentrations of AXER-204 before and after multiple treatments indicated that it does not accumulate in vivo.
Finally, the researchers also conducted an exploratory analysis of the therapeutic effect of AXER-204.
Of the 17 patients (single doses of 30 mg, 90 mg, or 200 mg AXER-204) who were evaluated for efficacy in Part 1, 5 (29%) had an increase of ≥3 points in the ISNCSCI UEMS score from baseline at Day 29 . None of the six patients who received a single 3 mg dose had an increase of ≥3 points.
In the second part, the AXER-204 group increased the ISNCSCI UEMS score from baseline by 1.5 points at day 169, which was higher than the 0.9 point of placebo, but the difference has not yet reached statistical significance (p=0.59) .
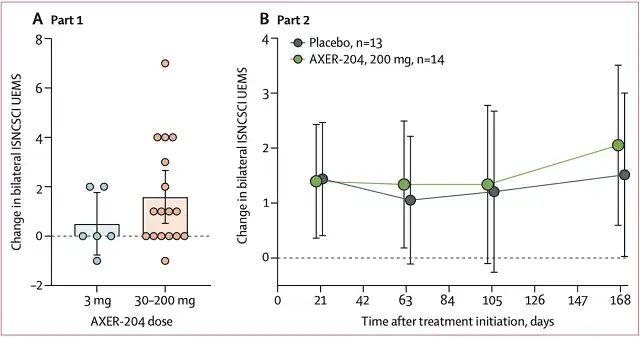
Treatment Effects in Two Parts of Subjects
Post hoc grouping in Part II of patients with incomplete spinal cord injury (American Spinal Injury Association Injury Scale, AIS B, C, D) who were not treated with AXER-204 (i.e. excluded participation in Part I) In the analysis, subjects in the AXER-204 group improved by 4 points on the ISNCSCI UEMS and by 9 points on the ISNCSCI Total Motor Score, while these measures were unchanged in the placebo group .
This suggests that patients with incomplete SCI are more likely than those with complete SCI to benefit from treatments that promote axon growth .
In addition, there may be an upper limit to the effect of AXER-204, resulting in subjects participating in the first part of the study receiving the vast majority of the benefit before the baseline measurement of the second part.
Of course, these are the researchers’ conjectures, and larger clinical studies are needed to explore whether AXER-204 can improve the prognosis of patients who meet these criteria.

AXER-204 was more effective in patients with incomplete spinal cord injury who were not treated with AXER-204
In general, this study is the first clinical study of chronic spinal cord injury that uses drugs to promote nerve repair, confirming that intrathecal injection of 200 mg of AXER-204 is safe and feasible, and no serious adverse events related to it were found.
Although the efficacy advantage of AXER-204 over placebo did not reach statistical significance in the second randomized, controlled study, it showed good results in AXER-204-naïve patients with incomplete spinal cord injury In the future, larger clinical studies are needed to confirm this.
At least, there is hope that the situation of no medicine available for spinal cord injury has been broken.
references
1. Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S et al: A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22(6):941-950 e946.
2.Ievins A, Moritz CT: Therapeutic Stimulation for Restoration of Function After Spinal Cord Injury. Physiology (Bethesda) 2017, 32(5):391-398.
3. Burton A: Expecting exoskeletons for more than spinal cord injury. Lancet Neurol 2018, 17(4):302-303.
4.Maynard G, Kannan R, Liu J, Wang W, Lam TKT, Wang X, Adamson C, Hackett C, Schwab JM, Liu C et al: Soluble Nogo-Receptor-Fc decoy (AXER-204) in patients with chronic Cervical spinal cord injury in the USA: a first-in-human and randomised clinical trial. Lancet Neurol 2023, 22(8):672-684.
5. Zheng B, Tuszynski MH: Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol 2023, 24(6):396-413.
6. GrandPre T, Nakamura F, Vartanian T, Strittmatter SM: Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000, 403(6768):439-444.
7.Wang X, Duffy P, McGee AW, Hasan O, Gould G, Tu N, Harel NY, Huang Y, Carson RE, Weinzimmer D et al: Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann Neurol 2011, 70 (5):805-821.
8.Wang X, Zhou T, Maynard GD, Terse PS, Cafferty WB, Kocsis JD, Strittmatter SM: Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain 2020, 143(6):1697- 1713.
The world first drug treatment of spinal cord injury: Clinic data released, patient benefit trend
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



