Targeting Iron-Dependent Cell Death in Tumor Immunotherapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Targeting Iron-Dependent Cell Death in Tumor Immunotherapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Targeting Iron-Dependent Cell Death in Tumor Immunotherapy.
Iron-dependent cell death, a recently discovered form of programmed cell death, plays a crucial role in tumor biology and therapy. This unique form of cell death, characterized by iron-dependent lipid peroxidation, is finely regulated by cellular metabolism networks involving lipids, iron, and amino acids.
Different tumors exhibit varying sensitivities to iron-dependent cell death. Recent evidence suggests that triple-negative breast cancer (TNBC), a highly aggressive and therapeutically challenging disease, is particularly susceptible to inducers of iron-dependent cell death, highlighting this novel form of non-apoptotic cell death as an attractive target for treating “hard-to-treat” tumors.
Interestingly, iron-dependent cell death has recently been associated with T cell-mediated anti-tumor immunity, impacting the effectiveness of tumor immunotherapy. Therefore, a better understanding of this iron-dependent cell death could lead to the discovery of new combination cancer therapy strategies with significant biological and clinical implications.
Molecular Mechanisms of Iron-Dependent Cell Death
Iron-dependent cell death is a regulated form of cell death induced by iron-dependent lipid peroxidation. Three key features of iron-dependent cell death have been elucidated: membrane lipid peroxidation, intracellular iron availability, and loss of antioxidant defense.
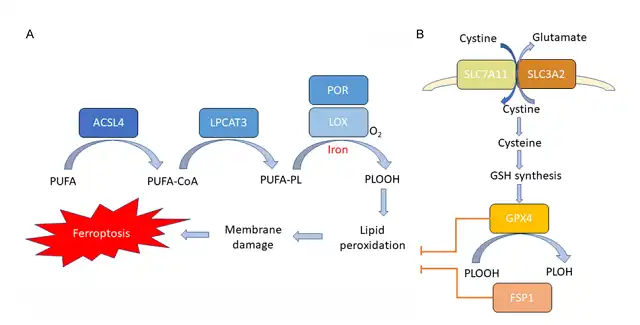
Lipid Peroxidation:
Lipid peroxidation leads to the disruption of the lipid bilayer and membrane damage, subsequently resulting in cell death. Cell membranes are rich in phospholipids (PLs) containing polyunsaturated fatty acids (PUFAs), which are highly susceptible to peroxidation induced by reactive oxygen species (ROS). The availability of PUFA-containing membrane PLs that can withstand peroxidation is crucial for iron-dependent cell death.
Polyunsaturated fatty acids need to be synthesized, activated, and incorporated into membrane PLs to participate in this death process, requiring two key enzymes, Acyl-CoA synthetase long-chain family member 4 (ACSL4), and lysophosphatidylcholine acyltransferase 3 (LPCAT3). ACSL4 catalyzes the attachment of long-chain PUFAs to coenzyme A (CoA), while LPCAT3 promotes esterification of these products and their integration into membrane phospholipids.
Certain lipoxygenases (LOXs) are considered major enzymes capable of directly oxidizing membrane lipids containing PUFAs. However, the mechanisms by which LOXs mediate iron-dependent cell death require further investigation. Another enzyme, cytochrome P450 oxidoreductase (POR), has recently been implicated in initiating lipid peroxidation.
Iron Accumulation:
As the name “iron-dependent cell death” suggests, iron is essential for the execution of this form of cell death. Iron is indispensable for the Fenton reaction, which generates free radicals and mediates lipid peroxidation. Additionally, iron is necessary for the activation of iron-containing enzymes LOX and POR, both responsible for oxidizing membrane PUFAs. Iron is also crucial for the oxidative-reductive metabolic processes involved in the generation of reactive oxygen species (ROS).
Because iron plays a critical role in the occurrence of iron-dependent cell death, cellular iron pools are intricately controlled by genes regulating intracellular iron storage, release, uptake, and export. Variations in cellular unstable iron influence sensitivity to iron-dependent cell death. For instance, an increase in iron input or degradation of iron storage proteins can enhance cellular iron utilization and sensitivity to iron-dependent cell death.
Loss of Antioxidant Capacity:
Under normal conditions, iron-mediated lipid oxidation is tightly regulated by the cellular antioxidant defense system. Glutathione peroxidase 4 (GPX4) is considered a key antioxidant enzyme, directly involved in eliminating hydrogen peroxide from the lipid bilayer and preventing the accumulation of lethal lipid ROS.
GPX4 utilizes glutathione (GSH) as a substrate to reduce lipid hydroperoxides in the membrane to harmless lipids. The synthesis of GSH is essential for GPX4 activity and requires three amino acids: cysteine, glycine, and glutamine. Cysteine is the rate-limiting substrate for glutathione synthesis and a crucial component of glutathione synthesis. The abundance of intracellular cysteine in mammalian cells is primarily regulated by two subunits of the xc system, SLC7A11 and SLC3A2. Small molecule inhibitors like erastin can inhibit cysteine uptake mediated by SLC7A11 and induce iron-dependent cell death in various cancers.
Recently, an alternative GPX4-independent mechanism of inhibiting iron-dependent cell death has been discovered. Iron-dependent cell death inhibitor protein 1 (FSP1)-CoQ system protects cells from GPX4 inhibition-induced iron-dependent cell death. FSP1 prevents lipid peroxidation by reducing lipid radicals. Therefore, cells utilize two pathways, cysteine-GSH-GPX4 and FSP1 CoQ axis, to inhibit lipid peroxidation and prevent iron-dependent cell death. When overwhelmed by iron-dependent lipid ROS accumulation, iron-dependent cell death occurs.
Iron-Dependent Cell Death in TNBC
Different types of cancer exhibit varying sensitivities to iron-dependent cell death. Recent evidence suggests that in TNBC, changes occur in the expression of genes related to iron-dependent cell death-associated metabolic pathways, including lipid, iron, and amino acid metabolism, making this therapy-resistant cancer particularly susceptible to iron-dependent cell death. The specific sensitivity of TNBC to iron-dependent cell death highlights the attractiveness of this non-apoptotic death pathway as a target for TNBC treatment.
Lipid Metabolism:
Dysregulated lipid metabolism can lead to lipid peroxidation and iron-dependent cell death, with ACSL4 being a critical component of the execution of iron-dependent cell death. Interestingly, studies have found that ACSL4 is preferentially expressed in TNBC compared to other types of breast cancer, and its expression predicts sensitivity to iron-dependent cell death. Recent research has also observed significant upregulation of ACSL4 in TNBC tumors and cell lines. Given that ACSL4 enriches cell membranes with long-chain PUFAs, this suggests that TNBC is rich in PUFAs and therefore particularly sensitive to iron-dependent cell death.
Iron Metabolism:
A sufficient intracellular iron pool is a prerequisite for executing iron-dependent cell death. Compared to normal cells, cancer cells exhibit higher iron dependency to promote growth. Recent research indicates that genes regulating intracellular iron levels are significantly upregulated in TNBC compared to non-TNBC tumors and cell lines. Notably, TNBC displays abundant low levels of iron export transporters, accompanied by high-level expression of iron import transferrin receptor. Changes in the expression of genes involved in iron metabolism regulation may contribute to increasing cellular labile iron pools, promoting iron-dependent lipid peroxidation, and making TNBC an iron-rich tumor susceptible to iron-dependent cell death.
Amino Acid Metabolism:
Amino acid metabolism is critical for the antioxidant defense system composed of cysteine uptake mediated by SLC7A11, GSH biosynthesis, and GPX4 activity. Cancer cells may exhibit altered reliance on specific amino acid metabolic pathways. Early studies found that TNBC, compared to other types of breast cancer, displayed a pronounced reliance on glutaminase metabolism necessary for supplementing SLC7A11, suggesting a potential connection between TNBC and iron-dependent cell death.
Additionally, compared to other tumor types, TNBC tumors show reduced expression of glut
athione synthetase (GSS), a key enzyme in GSH biosynthesis. The expression of GPX4 is also lower in TNBC compared to other types of breast cancer. Low intracellular GSH and GPX4 expression can weaken antioxidant defense capacity, increase lipid peroxidation, and render TNBC particularly sensitive to drugs that promote iron-dependent cell death.
Iron-Dependent Cell Death in Tumor Immunotherapy
Recently, it has been discovered that iron-dependent cell death contributes to the anti-tumor effects of CD8+ T cells and affects the outcomes of anti-PD-1/PD-L1 immunotherapy. Combining immunotherapy with approaches that induce iron-dependent cell death, such as radiation therapy and targeted therapy, can synergize to promote tumor control.
Combination with Immune Checkpoint Inhibitors and Cysteine Limitation:
Recent reports suggest that CD8+ T cells activated by anti-PD-L1 immunotherapy secrete IFN-γ after PD-L1 blockade, promoting iron-dependent cell death in tumor cells. Secreted IFN-γ significantly downregulates the expression of SLC3A2 and SLC7A11 in tumor cells, resulting in reduced cysteine uptake, enhanced lipid peroxidation, and subsequent iron-dependent cell death. The combination of cysteine/cystine deprivation and anti-PD-L1 has the potential to generate effective anti-tumor immunity by inducing iron-dependent cell death.
Combination with Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors:
Recent research indicates that resistance to anti-PD-L1 therapy can be overcome by combining it with tyrosine kinase inhibitor (TKI) TYR03, which promotes iron-dependent cell death. Increased TYR03 expression has been observed in anti-PD-1-resistant tumors. Mechanistically, the TYR03 signaling pathway upregulates key iron-dependent cell death genes like SLC3A2, thereby inhibiting tumor cell iron-dependent cell death. In a TNBC mouse model resistant to anti-PD-1 therapy, inhibiting TYR03 promoted iron-dependent cell death and sensitized tumors to anti-PD-1 therapy. This study reveals that inhibiting iron-dependent cell death through TYR03 inhibition is an effective strategy for overcoming immunotherapy resistance.
Combination with Radiation Therapy:
Recent evidence suggests that the synergy between radiation therapy and immunotherapy is linked to increased sensitivity to iron-dependent cell death. Radiation has been shown to induce iron-dependent cell death, and genetic and biochemical features of iron-dependent cell death have been observed in cancer cells after radiation treatment. The mechanisms involve radiation-induced ROS generation and upregulation of ACSL4, leading to enhanced lipid synthesis, increased lipid peroxidation, and subsequent membrane damage. Therefore, the anti-tumor effects of radiation therapy can be attributed not only to cell death induced by DNA damage but also to iron-dependent cell death induction. Radiation therapy in combination with immunotherapy downregulates SLC7A11 through DNA damage-activated kinase ATM and IFN-γ mediation, resulting in reduced cysteine uptake, increased iron-dependent cell death, and enhanced tumor control. These studies reveal that iron-dependent cell death is a novel mechanism of synergy between immunotherapy and radiation therapy.
Combination with Immune Checkpoint Inhibitors and T Cell Iron-Dependent Cell Death Inhibitors:
Apart from inducing tumor cell iron-dependent cell death, T cells themselves can undergo iron-dependent cell death, which may attenuate their immune response. T cells lacking GPX4 rapidly accumulate lipid peroxides in the membrane and undergo iron-dependent cell death. Similar to cancer cells, ACSL4 is essential for iron-dependent cell death in CD8+ T cells and their immune function.
Recently, two studies have shown increased expression of CD36 in CD8+ tumor-infiltrating lymphocytes (TILs). Inherent CD36 in T cells promotes the uptake of oxidized lipids and induces lipid peroxidation, leading to dysfunction in CD8+ T cell function. These findings reveal CD8+ T cell iron-dependent cell death as a new mode of tumor immune suppression and emphasize the therapeutic potential of blocking CD36 to enhance anti-tumor immunity. Importantly, these studies also suggest that GPX4 plays a role in regulating the anti-tumor function of CD8+ TILs. Therefore, therapeutic induction of iron-dependent cell death in cancer cells through GPX4 inhibitors may result in unnecessary off-target effects on T cells and produce adverse toxicity.
Conclusion:
Iron-dependent cell death, driven by lipid oxidation of PUFAs, intracellular iron accumulation, and loss of antioxidant defense, plays a regulatory role in various cancer types, including TNBC. Specifically, TNBC exhibits a unique expression pattern of genes related to iron-dependent cell death, making it particularly susceptible to inducers of iron-dependent cell death. Therefore, targeting iron-dependent cell death holds promise as a treatment strategy for this therapy-resistant cancer.
Furthermore, iron-dependent cell death plays a crucial role in T cell-mediated anti-tumor immunity, impacting the efficacy of immunotherapy. By directly or indirectly inducing iron-dependent cell death, such as through radiation therapy and targeted therapy, novel combinations with immunotherapy offer hope for enhancing anti-PD-1/PD-L1 immunotherapy. Thus, it is essential to explore further strategies for modulating iron-dependent cell death in combination with immunotherapy. These discoveries expand our understanding of this new form of cell death and provide new opportunities for future research directions.
Reference:
1. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res. 2021; 11(12): 5856–5863.
Targeting Iron-Dependent Cell Death in Tumor Immunotherapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



