Highest Liver Cancer Risk in Chronic Hepatitis B Patients with this Viral Load Range
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Highest Liver Cancer Risk in Chronic Hepatitis B Patients with this Viral Load Range
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
GUT: Alert! Highest Liver Cancer Risk in Chronic Hepatitis B Patients with this Viral Load Range
Chronic hepatitis B (CHB) affects approximately 296 million individuals worldwide, with about 70 million carriers of the hepatitis B virus (HBV) in China alone.
Among them, 20 to 30 million are diagnosed with chronic hepatitis B, which is a major contributor to hepatocellular carcinoma (HCC), a form of liver cancer.
Based on antigen and serological characteristics, chronic hepatitis B can be categorized into four phases: immune tolerance, immune activation, immune control, and reactivation.
Previous research has shown that long-term antiviral therapy can reduce the risk of developing liver cancer by 50% in chronic hepatitis B patients in the immune activation phase.
However, even patients undergoing treatment remain at risk of developing cancer because once antiviral therapy begins, the serum HBV DNA levels in chronic hepatitis B patients can rapidly change and become challenging to monitor accurately.
Some experts believe that the baseline HBV DNA levels cannot predict the risk of cancer in patients undergoing treatment.
Nevertheless, recent research has revealed that for hepatitis B e-antigen (HBeAg)-positive chronic hepatitis B adults without cirrhosis and with baseline HBV DNA levels ≥5 log10 IU/mL, the baseline HBV DNA level is a significant independent risk factor negatively associated with cancer risk during antiviral treatment.
Recently, GUT published a study indicating that patients with a moderate baseline HBV viral load, especially those with HBV DNA levels around 6 log10 IU/mL, continue to have a high risk of developing liver cancer despite receiving long-term antiviral therapy.
In clinical practice, it is advisable to initiate antiviral treatment early based on the patient’s baseline viral load to minimize the risk of liver cancer in non-cirrhotic adult hepatitis B patients.

Screenshot source: GUT
This study was a multicenter historical cohort study that included 4,693 adult chronic hepatitis B patients from five tertiary medical centers in South Korea between January 2007 and December 2019. The patients had a median age of 45 years, 61.2% were male, and the median HBV DNA level was 7.0 log10 IU/mL. None of the patients had cirrhosis, and they were treated with either entecavir or tenofovir disoproxil fumarate (TDF) as antiviral therapy, with the baseline HBV viral load used as a classification criterion. At baseline, all patients had been hepatitis B surface antigen (HBsAg) positive for more than 6 months, were either HBeAg-negative or -positive, and had an HBV DNA level of at least 2000 IU/mL.
Based on the baseline HBV DNA levels, the patients were divided into three groups: low viral load (HBV DNA levels ranging from 3.30 to 4.99 log10 IU/mL, 725 cases), moderate viral load (HBV DNA levels ranging from 5.00 to 7.99 log10 IU/mL, 2,536 cases), and high viral load (HBV DNA levels ≥8.00 log10 IU/mL, 1,432 cases). The index date was defined as the start of entecavir or TDF treatment, and patients received bi-annual liver cancer ultrasound screening and alpha-fetoprotein monitoring from the index date until they developed liver cancer, died, underwent a liver transplant, or had their last follow-up (December 31, 2021).
Patients in the moderate viral load group had the highest incidence of hepatocellular carcinoma
Patients with a moderate viral load had the highest incidence of liver cancer during the study period. The primary outcome was the incidence of liver cancer. During a median follow-up of 7.6 years under continuous antiviral therapy, 193 patients developed liver cancer (incidence rate of 0.53 per 100 person-years), and 66 patients died or underwent liver transplantation (incidence rate of 0.18 per 100 person-years). Survival analysis showed that the order of liver cancer incidence from highest to lowest was: moderate viral load group > low viral load group > high viral load group (P < 0.001).
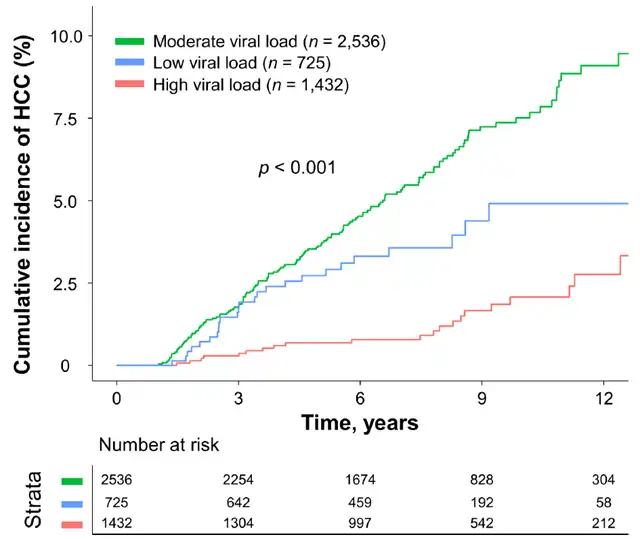
Cumulative incidence of hepatocellular carcinoma during treatment in chronic hepatitis B patients grouped according to baseline HBV DNA levels (Picture source: Reference [1])
(Green is the medium viral load group, blue is the low viral load group, and orange is the high viral load group)
The article provides three potential explanations for these results:
-
Most HBeAg-positive chronic hepatitis B patients are in the initial immune tolerance phase of infection, with very high HBV DNA levels (≥8.00 log10 IU/mL) because each milliliter of serum can contain 10^9 to 10^10 viruses, as liver cells completely infected with the virus can produce a large number. In contrast, low-level cytotoxic T lymphocytes can gradually eliminate infected liver cells, leading to the development of liver cell clones that produce small amounts of virus and exhibit resistance to HBV infection. This is a major risk factor for liver cancer. Therefore, a reduction in HBV DNA viral load (<8.00 log10 IU/mL) may indicate an increase in the number of drug-resistant clonal liver cells and an increased risk of liver cancer.
-
During the process of HBV DNA integration into the human chromosome, it can disrupt tumor suppressor genes or activate pro-oncogenes involved in the development of liver cancer, which occurs in the early stages of infection.
-
In chronic hepatitis B patients with normal alanine aminotransferase levels and no significant fibrosis, a moderate viral load (5.00 to 7.00 log10 IU/mL) has been confirmed as a risk factor for liver inflammation, which is itself a risk factor for liver cancer.
Relationship between baseline HBV DNA levels and liver cancer risk
Statistical analysis indicates that when the baseline HBV DNA level decreases to below 8.00 log10 IU/mL, the risk of liver cancer sharply increases.
The risk of liver cancer peaks when the HBV DNA level drops to the range of 6.00 to 6.99 log10 IU/mL. Further reduction of the HBV DNA level to below 6.00 log10 IU/mL is associated with a nonlinear parabolic increase in liver cancer risk.
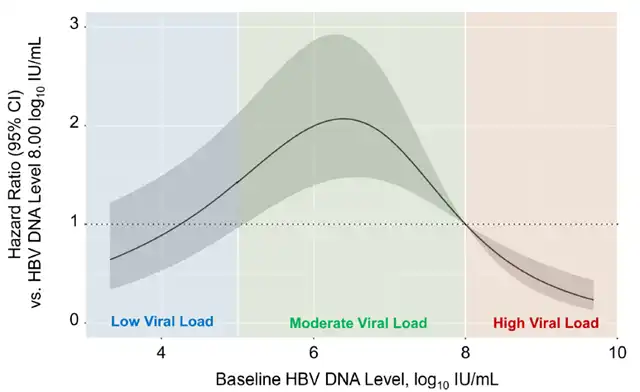
Chronic hepatitis B patients receiving antiviral treatment, the risk ratio of liver cancer during treatment adjusted according to the baseline HBV DNA level (Picture source: Reference [1])
(The blue color block represents low viral load, the green color block represents medium viral load, and the orange color block represents high viral load)
Considering that patients with a moderate viral load have the highest risk of developing liver cancer, the researchers further subdivided this group based on their HBV DNA levels into 5.00-5.99 log10 IU/mL, 6.00-6.99 log10 IU/mL, and 7.00-7.99 log10 IU/mL categories. The statistical analysis results show that when the HBV DNA level is in the range of 6.00-6.99 log10 IU/mL, the patients still have the highest risk of developing liver cancer (HR=3.36, 95% CI: 2.00-5.65, P < 0.001). These results are more pronounced in HBeAg-positive patients, younger patients, or those with less severe liver fibrosis.
Patients not receiving antiviral therapy are at higher risk of developing hepatocellular carcinoma
Patients who did not receive antiviral treatment had a higher risk of developing liver cancer compared to matched patients who did receive antiviral treatment, regardless of the viral load level. Among those who received antiviral treatment, patients with a moderate viral load had a significantly higher reduction in their cancer risk compared to those with a high viral load.
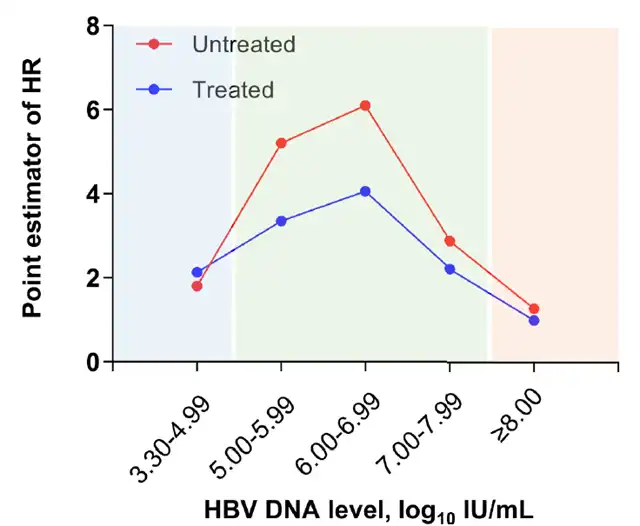
Adjusted HR of liver cancer risk for all patients (Picture source: Reference [1])
(The blue color block represents low viral load, the green color block represents medium viral load, and the orange color block represents high viral load; red dots represent patients who have not received treatment, and blue dots represent patients receiving antiviral treatment)
Summary
In summary, patients with a moderate baseline HBV viral load, particularly those with HBV DNA levels around 6 log10 IU/mL, still face a high risk of developing liver cancer despite receiving long-term antiviral therapy.
In clinical practice, it is essential to initiate antiviral treatment early based on the patient’s baseline viral load to minimize the risk of liver cancer in non-cirrhotic adult hepatitis B patients.
GUT: Alert! Highest Liver Cancer Risk in Chronic Hepatitis B Patients with this Viral Load Range
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



