TANGO Study: Challenges in Tau Antibody Development for Alzheimer’s
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
TANGO Study: Challenges in Tau Antibody Development for Alzheimer’s
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
TANGO Study: Challenges in Tau Antibody Development for Alzheimer’s
The TANGO study data on the treatment of Alzheimer’s disease (AD) has been released, indicating that targeting free Tau protein may indeed be ineffective.
As we know, the pathological features of Alzheimer’s disease include the abnormal accumulation or aggregation of extracellular β-amyloid protein (Aβ) and intracellular hyperphosphorylated Tau protein.
This year, Aβ antibody drugs have made significant progress. The first Aβ antibody drug, Lecanemab, has been FDA approved for the treatment of adult AD. It selectively binds and removes insoluble Aβ aggregates, slowing down the Clinical Dementia Rating Sum of Boxes (CDR-SB) scores by 27% in early AD patients [1], marking a breakthrough in molecular targeting for AD treatment. Another Aβ antibody drug, Donanemab, also reported phase 3 clinical trial results this summer [2], showing efficacy similar to Lecanemab and expected FDA approval soon.
In contrast, Tau antibody drugs have faced repeated setbacks in their development journey. However, the silver lining is that lessons have been learned.
Targeting free Tau protein may indeed be ineffective!
Recently, the phase 2 clinical trial data of the Tau antibody drug Gosuranemab, named TANGO (NCT03352557), were published in the journal “Nature Aging” [3]. The results indicate that while Gosuranemab effectively reduces Tau protein levels in cerebrospinal fluid and demonstrates good safety and tolerability, it is ineffective in participants with AD-related mild cognitive impairment (MCI) or mild AD compared to the placebo.
For those following developments in the AD field, it is known that this clinical trial had already declared failure in 2021, leading to the termination of Gosuranemab’s development for AD [4]. Nevertheless, failure does not mean loss of hope, and with the official release of the data, let’s explore the challenges facing Tau antibody drugs.

Gosuranemab is a humanized IgG4 monoclonal antibody targeting the N-terminus of Tau protein, capable of strongly binding and clearing free Tau protein from cerebrospinal fluid. In theory, targeting the clearance of free Tau protein could prevent its uptake by neurons or its spread between neurons, thereby reducing the accumulation of abnormal Tau protein in neurons and alleviating early AD progression.
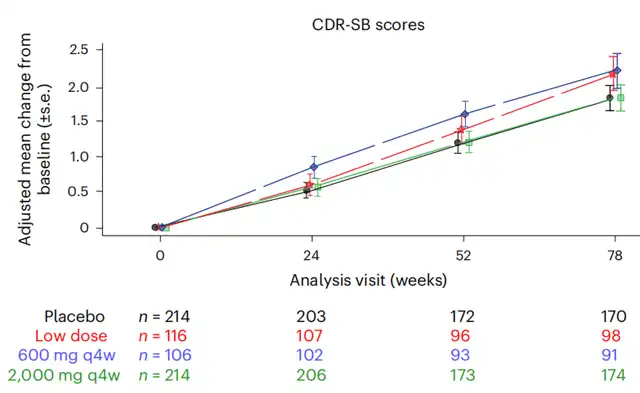
In the TANGO clinical trial, 650 participants with AD-related MCI (303) or mild AD (347) received different doses of Gosuranemab or placebo treatment.
The results confirm the previously reported good safety and tolerability of Gosuranemab in all dose groups. However, participants treated with any dose of Gosuranemab did not show improvement in Tau protein aggregation in neurons compared to the placebo group, as indicated by positron emission tomography (PET) scans. Moreover, no statistically significant treatment benefits were observed in clinical dementia rating sum of boxes (CDR-SB) scores, Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), Activities of Daily Living (ADCS-ADL), Mini-Mental State Examination (MMSE), Functional Activities Questionnaire (FAQ), and other exploratory endpoints at 78 weeks compared to the placebo.
Numerous studies suggest that Aβ begins to aggregate decades before AD symptoms appear, reaching a plateau by the time symptoms are significant. In contrast, the accumulation level of Tau protein is closely related to the progression of AD symptoms. Therefore, many believe that targeting Tau protein pathology is more effective in slowing early AD progression than targeting Aβ plaques.
However, clearing Tau protein is not an easy task. Firstly, unlike Aβ, which is located extracellularly, most Tau proteins are located inside neurons and are not easily bound by antibodies. Secondly, compared to Aβ, Tau protein’s post-translational modifications are more diverse, making antibody design more challenging.
Neurons can take up Tau protein from cerebrospinal fluid or release Tau protein to another neuron. As mentioned earlier, the strategy of targeting free Tau protein aims to seize this opportunity and limit the spread of Tau protein.
In a commentary article published alongside this paper, it is noted that Gosuranemab, along with other antibodies targeting free Tau protein such as Zagotenemab, Semorinemab, and Tilavonemab, has consecutively failed. This suggests that merely restricting the level of free Tau protein in cerebrospinal fluid may not solve the problem, and effective reduction of abnormal Tau protein aggregation in neurons may be crucial [5].
Of course, even if antibodies targeting Tau protein aggregation in neurons emerge in the future, whether they can translate into clinical benefits is still a question. Some argue that the excessive phosphorylation and entanglement of Tau protein are results of AD pathology, such as Aβ plaques, rather than driving factors. The feasibility of molecular therapy targeting Tau protein remains to be seen, and it may depend on the emergence of drugs truly targeting aggregated Tau protein [5].
TANGO Study: Challenges in Tau Antibody Development for Alzheimer’s
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



