Development of antibody drugs: GPCR antibody drugs
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Development of antibody drugs: GPCR antibody drugs
Development of antibody drugs: GPCR antibody drugs. GPCR (G Protein-Coupled Receptor), or G protein-coupled receptor, is the largest membrane protein family in the mammalian genome.
It is widely distributed in the central nervous system, immune system, cardiovascular, retina and other organs and tissues, and participates in the body’s Development and normal function exercise. If the related intracellular pathway regulation is abnormal, or foreign pathogens use it as a receptor to attack the body’s cells, a series of diseases will occur. Therefore, GPCR is regarded as an important drug development target, and GPCR antibody drug development has very important value.
Introduction to GPCR
The main body of the GPCR is composed of 7 segments of alpha helix structure that spans the plasma membrane of the cell. The N-terminal and three loops are located outside the cell and participate in the interaction between the receptor and its ligand; the C-terminal and three loops are located in the cell, and the C-terminal and the third loop are located in the interaction between the GPCR protein and the downstream G protein to mediate It plays an important role in the signal transduction process in the cell. The binding of specific ligands to GPCRs will cause the activation of G protein, produce the second messenger Ca2+ or cAMP, and transmit the extracellular signals received by the GPCR downstream; but GPCRs can also mediate signal transduction independent of G protein, such as It regulates downstream pathways by interacting with β-arrestin and other molecules.
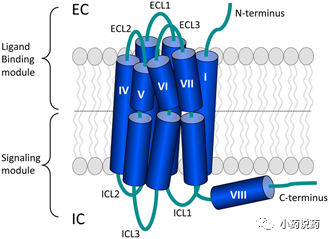
For members of the GPCR superfamily, there are a variety of different classification methods, two of which are more popular: one is the AF classification system for GPCR proteins in all organisms; the other is based on sequence similarity and functional similarity. GPCRs are divided into 5 categories (abbreviated as GRAFS): Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, Secretin, etc. Most human GPCRs can be classified among them.
The Rhodopsin receptor family (ClassA) is the largest family in the GPCR superfamily. For most members, the main structural feature is that the N-terminal is short, and their natural ligands directly bind to the transmembrane region or indirectly affect their conformation by binding to the extracellular loop structure. The types of ligands are very wide, including peptides, amines, purines and so on. This family can be further divided into α, β, γ, and δ subcategories. The members of the alpha subclass include a variety of important receptors for amines and small molecule compounds, including histamine receptors, dopamine receptors, cannabinoid receptors, and so on. The β subclass contains a variety of polypeptide receptors, such as endothelin receptors, oxytocin receptors and so on. One of the more concerned receptors in the gamma subclass is the chemokine receptor family, and other members include some chemokine receptors, neuropeptide receptors and so on. The delta subclass includes some glycoprotein receptors and olfactory-related receptors. Generally speaking, the members of the Rhodopsin receptor family are relatively complex, and it is difficult to classify them based on single characteristics such as structure, function, and expression distribution.
The structural feature of class B receptors is that they have a large extracellular region. The Secretin receptor family (ClassB) has been found to have 15 members, all of which are polypeptide hormone receptors. The polypeptide binds to the polypeptide binding region of its extracellular domain to activate the receptor; and most of the Adhesion receptor family (ClassB) The members have not been found to have natural ligands.
The Glutamate receptor family (ClassC) includes 8 metabolic glutamate receptors (GRMs), 2 GABA receptors (GABABRs), calcium sensitive receptors (CASR), sweet and umami taste receptors (TAS1R1- 3), GPRC6A and some orphan receptors. Most members have the binding region of endogenous ligand at their N-terminus.
The Frizzled/TAS2 receptor family can be subdivided into two subfamilies: one is the frizzled and smoothened receptors (ClassF), which contains 10 frizzled receptors (FZD1-10) and 1 smoothened receptor (SMO). Wnt glycoprotein is a natural ligand, and the signal pathway regulation of the latter seems to be receptor-independent; the second is thetaste2 receptors, mainly bitter taste receptors, and some are still orphan receptors.
The relationship between GPCR protein and disease
The GPCR family is widely distributed in the human body and has complex functions. Therefore, it is related to the occurrence and progression of many diseases, or plays an important role in it. According to the clearer studies so far, the diseases involved in GPCRs are mainly divided into three categories: cancer, inflammation, and cardiovascular/metabolic diseases.
GPCR and cancer: Studies have shown that various members of the GPCR family are involved in the occurrence and progression of various cancers. Hormone receptor GPCRs are involved in certain hormone-dependent cancers. Some proteases activate receptors such as PAR1, which activate and activate downstream signaling pathways under the catalysis of MMP-1 to promote the invasion and deterioration of cancer cells. Some chemokine receptors, such as CXCR2 and CXCR4, are highly expressed in myeloma and lymphoma cells, and may also be highly expressed on solid tumor cells such as pancreatic cancer, and participate in cell migration and angiogenesis.
GPCR and inflammation: The chemokine receptor family is mainly expressed on various cells of the immune system, and participates in the development, migration, survival, and immune function of immune cells and other physiological and pathological processes. Among them, the inflammatory response is a type of pathological phenomenon caused by the activation of chemokine receptors on immune cells to mediate immune cells to exert the host’s immune function. For example, CXCR1 and CXCR2 are mainly expressed on neutrophils. When infection or damage occurs somewhere in the body, neutrophils will migrate to the lesion under the chemotaxis of the corresponding chemokines (CXCL1, CXCL2, CXCL8) and reach Inflammatory factors are released after the lesion, thereby producing a local inflammatory response.
GPCR and cardiovascular/metabolic diseases: GPCR members related to cardiovascular diseases are mainly distributed in the α subclass of the ClassA family, such as one of the most important neurohumoral regulation systems in the human body, AT1R and AT2R in the angiotensin system (RAAS) And Mas-R. Activation of AT1R receptor causes vasoconstriction, cell proliferation and migration, inflammatory fibrosis, myocardial remodeling and hypertrophy, sodium and water retention, and ROS generation, etc.; while activation of Mas receptor leads to vasodilation, inhibits cell proliferation and migration, anti-inflammatory fibrosis, Anticoagulation, promotion of EDRF, NO production, inhibition of myocardial hypertrophy and growth, etc., the two have mutually antagonistic effects and jointly maintain the body’s homeostasis.
GPCR members related to metabolic diseases are mainly fatty acid receptors distributed in the ClassA family, such as GPR120, GPR41 and GPR43, and the Secretin receptor family in the ClassB family, including GLP-1R and GCGR. Food nutrients can be decomposed in the intestine to produce free fatty acids. In addition to being absorbed, oxidized and decomposed to produce energy for use by the body, free fatty acids can also activate signaling pathways by binding to fatty acid receptors and participate in the regulation of various physiological functions, such as maintenance Energy balance, metabolic homeostasis, regulation of lipid formation and decomposition, monitoring of bacterial populations, etc.
Glucagon-like peptide 1 (GLP-1) activates GLP-1R to increase the level of insulin secretion in pancreatic β-cells, thereby controlling blood sugar; at the same time, it also makes people feel full, slows down intestinal peristalsis, and reduces food intake . Glucagon (GCG) is activated by binding to its receptor GCGR, enhances liver glycogen degradation, regulates the de novo synthesis pathway of glucose, resists the hypoglycemic effect of insulin, and raises blood sugar. In patients with type 2 diabetes, hyperglycemia is accompanied by hyperglucagonism.
In addition to the main diseases of these three types of diseases, there are other diseases that have been shown to be related to GPCRs, such as: CCR5 is involved in the invasion of HIV into T cells and is an important target for the treatment of AIDS; such as calcitonin-related gene peptide receptor CGRPR and its ligand CGRP are expressed in trigeminal neurons, and the downstream signaling pathways activated by the interaction of the two have been shown to be related to migraine.
Current status of GPCR antibody drug development
It is currently believed that there are about 370 GPCRs that can become drug targets. For GPCR targets, the main effect of antibody drugs is to block the activation of the target by extracellular signals and regulate the intracellular signal transduction mediated by the target. Compared with small molecules, the clearance rate of antibodies in the body is lower, the action time is longer, and the corresponding administration frequency is lower; and at a given dose, the individual difference in the plasma concentration of antibodies is smaller.
The special advantages of antibody drug therapy for GPCRs can be summarized into the following three aspects:
The first is the unique druggability of antibody drugs. Antibodies can block the binding of GPCRs to ligands, allow GPCRs to be endocytosed, and reduce or terminate downstream signals of GPCRs. It can also bind to specific epitopes of GPCRs, stabilize the specific conformation of GPCRs when activated, and produce preferential biological activities.
The second is the good selectivity of antibody drugs. Drugs that bind to low-conserved sites between receptors have good specificity. For example, members of the Secretin receptor family have a longer extracellular N-terminus, while the sequence differences between similar members are mainly concentrated at the N-terminus; while the transmembrane region where small molecules bind is relatively conservative.
Then there is the distribution of antibody drugs in the body. Many GPCRs are expressed in the peripheral and central nervous system, and the ability of antibody drugs to penetrate into the central nervous system is restricted due to the barrier of the blood-brain barrier. Therefore, for GPCRs that are expressed in the peripheral and central nervous system, if you only need to target Part of the peripheral design drugs can develop therapeutic antibodies, so that the drugs are mainly distributed in the peripheral area and reduce the toxic and side effects on the central nervous system.
Mogamulizumab
Mogamulizumab is the first monoclonal antibody that has been approved to be marketed targeting GPCRs. Mogamulizumab was developed by Kyowa Hakko Kirin Co., Ltd. (Kyowa Hakko Kirin), and was approved by the PMDA in Japan on March 30, 2012 and sold in the Japanese market under the trade name Poteligeo®. Mogamulizumab is a humanized monoclonal antibody targeting CC chemokine receptor 4 (CCR4), which is a G protein-coupled receptor (GPCR) expressed on regulatory T cells and helper T cells. The approved indications for the drug are CCR4-positive adult T-cell leukemia, CCR-positive peripheral T-cell lymphoma, and CCR4-positive cutaneous T-cell lymphoma. Poteligeo® is a solution for intravenous infusion. Each bottle contains 20mg Mogamulizumab in a total of 5mL. The recommended dose is 1 mg/kg each time, once a week, for a total of 8 times.
Erenumab/fremanezumab/galcanezumab
2018 was a very lively year for GPCR antibody drugs. Three consecutive drugs targeting the same GPCR target were approved by the FDA for marketing. Novartis/Amgen’s Erenumab is the first preventive migraine treatment drug approved by the FDA. It was approved for marketing in the United States in May 2018 under the trade name Aimovig; Teva’s Ajovy (fremanezumab) was approved by the FDA in September 2018. Emgality (galcanezumab) from Eli Lilly was approved in the same month. The above three drugs are monoclonal antibodies that target CGRP or its receptors. They are all administered by subcutaneous injection and can be operated by the patient themselves, but the injection frequency of the three is different. Among them, Aimovig and Emgality require monthly injections. Once, but Ajovy can choose once a month or once every quarter. There has never been an effective treatment in the field of migraine, and it has suddenly entered a state of competition among giants.
In addition, there are a number of drugs in different stages of development.
It can be seen that in the research pipeline, the types of targets and indications are relatively diverse. There are two main companies developing GPCR antibody drugs in China:
The REMD-477 under development by Beijing Kexinmeide is an inhibitory antibody against the glucagon receptor GCGR. By inhibiting GCGR, it reduces glucagon signaling on liver cells, thereby inhibiting liver glycogen degradation and de novo synthesis of endogenous glucose. At present, the project is in the second phase of clinical research for diabetes indications.
Fortune Huaning (Hangzhou) Biopharmaceutical Co., Ltd. is developing two GPCR antibody drugs, each for 3 indications. GMA102 injection (Glutazumab) is an ultra-long-acting GLP-1 receptor agonist independently developed by Hongyun Huaning. It is formed by the fusion of a humanized antibody targeting GLP-1 receptor and a GLP-1 fragment. The clinical indications are Type 2 diabetes (GMA102) and obesity (GMA105). In the early clinical trials of Ib/IIa carried out in Australia, it showed good blood sugar and weight reduction effects, and was significantly better than existing similar products in safety and tolerability. It is expected to become a new generation of ultra-long-acting hypoglycemic ( 2 weeks or even once a month) and weight loss (1 week or even once a month) treatment drugs. In view of the good clinical results in the early stage, GMA102 is currently planning to directly carry out phase III clinical trials in China, and this trial will serve as a key clinical trial for GMA102’s global marketing application.
GMA301 injection (Getagozumab) is the world’s first monoclonal antibody drug targeting endothelin receptor (ETa) independently developed by Hongyun Huaning. Its clinical indication is pulmonary hypertension. In 2017, it was recognized as a rare disease drug (orphan drug) by the US FDA. In the early phase Ia clinical trials conducted in Australia, it showed extremely superior safety over the existing ETa-blocking small molecule drugs. At present, the drug has been approved for clinical trials in China and the United States at the same time.
Based on the good results of the Australian Phase Ia clinical trial, China and the United States Drug Administration have agreed that the drug will omit clinical trials in healthy people and directly carry out Phase Ib clinical trials for patients with pulmonary hypertension. test. The drug breaks through the treatment pattern where there is no biological antibody drug in this field. According to the results of preclinical cynomolgus monkey animal experiments, GMA301 is expected to transform pulmonary hypertension, a terminal illness in the cardiovascular field with an average survival period of no more than 3 years, into a long-term disease. Controlled chronic diseases.
In addition, because children, especially young children, have inherent difficulties in oral administration, and the small molecule drugs of similar ETa inhibitors are mostly in oral dosage forms, GMA301 injection has inherent advantages. This drug has recently applied to the FDA for pediatric orphan drug qualification Identified. In addition to these two drugs, Hongyun Huaning also has several GPCR-based Bibody developments, which are in the stage of early discovery and preclinical research.
As an important class of drug target molecules, GPCRs are attracting more and more attention in the field of drug development, especially in the field of monoclonal antibody drug development. More and more clinical drug experiments have been extended to antibody drugs targeting GPCRs. Human understanding of the biological properties of GPCRs in oncology, especially tumor immunology, has greatly promoted the development of targeted therapeutic drugs in this field. The application of new generation protein drug development strategies, such as bispecific antibodies and antibody-conjugated drugs, has also promoted the advancement of this field. The development of bispecific antibodies based on GPCR antibodies will open up a new field for the application of antibody drugs.
(source:internet, reference only)
Disclaimer of medicaltrend.org



