Allosteric sites at the GPCR-lipid membrane interface
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Allosteric sites at the GPCR-lipid membrane interface
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Allosteric sites at the GPCR-lipid membrane interface.
Traditional GPCR targeted drugs mainly bind to highly conserved orthomorphic sites on the receptor. Some drugs have achieved some successful applications.
Molecular design
Drug molecular design is the sway of thinking in drug discovery. Let us taste the works of drug molecular designers together. It takes time for finished drugs to be marketed. Original design is wonderful.
As a superfamily of membrane receptors, G protein-coupled receptors play an important role in physiological processes, including cell proliferation, metabolism and neuromodulation.
GPCRs are important targets of therapeutic drugs, and allosteric regulation of GPCRs is a new direction for drug discovery.
Traditional GPCR targeted drugs mainly bind to highly conserved orthomorphic sites on the receptor.
Although some drugs have achieved some successful applications, the high degree of conservation of orthomorphic drug targets leads to adverse reactions in the process of drug application.
Compared with normal ligands, allosteric modulators (also called allosteric ligands) can reduce side effects by targeting less conserved allosteric sites in the receptor.
Due to major breakthroughs in structural biology in recent years, allosteric sites in the extracellular, intracellular, or 7 transmembrane spiral core regions of GPCRs have been reported successively.
However, the traditional GPCR allosteric sites also have a certain degree of conservation among homologous subtypes, making the application of GPCR allosteric modulators a problem.
A promising way to solve this problem is to design allosteric modulators that target the receptor surface position.
However, the allosteric sites on the outside of the cell are usually flat, making design very difficult.
Interestingly, in recent years, a unique type of allosteric site-the receptor-lipid bilayer interface (Lipid-Facing) allosteric binding site has been continuously discovered.
This kind of allosteric site is different from the traditional allosteric site.
The point is very different, and it is more dependent on lipid bilayers than traditional allosteric sites.
So far, this type of site is unique to GPCRs, showing diversity in specific receptors, and is a promising strategy to overcome the difficulties of GPCR drug discovery.
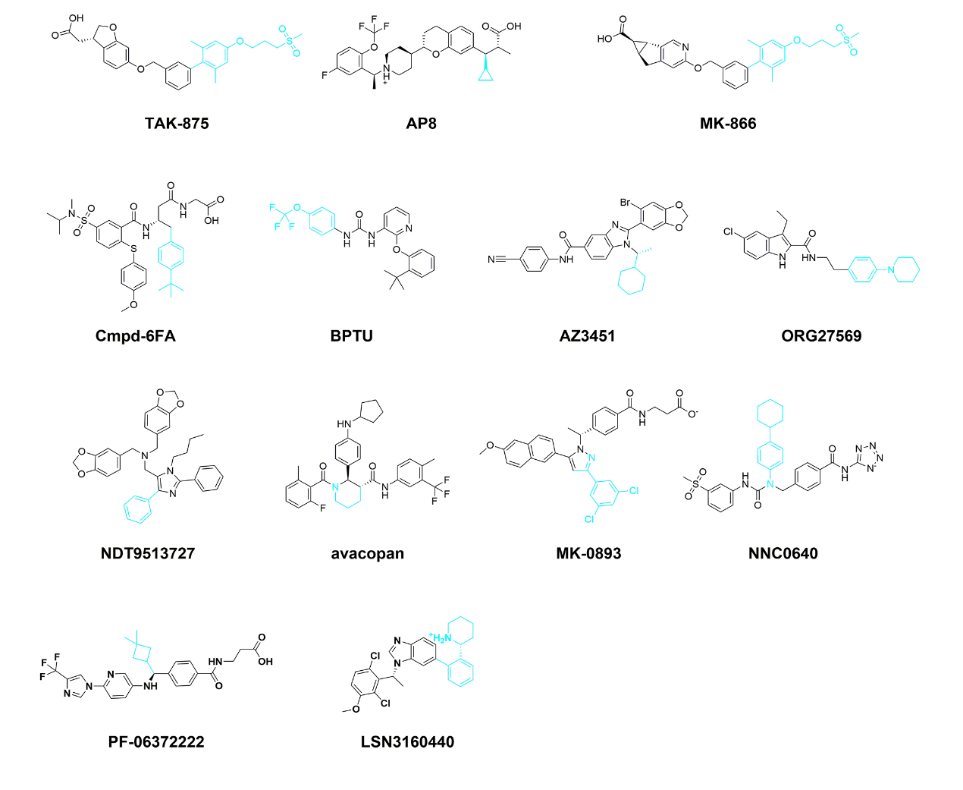
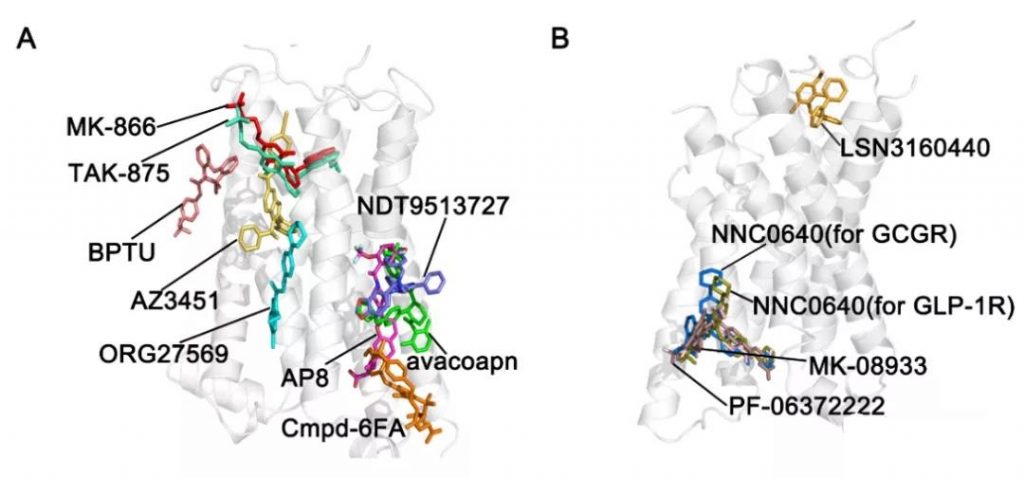
Here we summarize the research progress of this new allosteric site in recent years. So far, this new allosteric site has only been found in class A and class B GPCRs (including 6 class A and 2 class B), and there are 13 types of small molecules targeting this site.
We focused on the interaction between small molecule modulators and lipid bilayers, and the boundary area to inspire the development of allosteric ligands for such allosteric sites.
A. Lipid-Facing allosteric binding sites of class A GPCRs
| GPCR | Allosteric modulator | PDB | Resolution(Å) | Allosteric site |
| Class A | ||||
| GPR40 | TAK-875 | 4PHU | 2.33 | outside 7TMD (III-V), extracellular side |
| GPR40 | AP8 | 5TZY | 3.22 | outside 7TMD (II-V), intracellular side |
| GPR40 | MK-866 | 5TZR | 2.2 | outside 7TMD (III-V), extracellular side |
| β2AR | Cmpd6FA | 6N48 | 3.2 | outside 7TMD (II-IV), intracellular side |
| CB1 | ORG27569 | 6KQI | 3.245 | outside 7TMD (II-IV), intracellular side |
| P2Y1R | BPTU | 4XNV | 2.3 | outside 7TMD (I-III), intracellular side |
| PAR2 | AZ3451 | 5NDZ | 3.6 | outside 7TMD (II-IV), extracellular side |
| C5aR | NDT9513727 | 5O9H | 2.7 | outside 7TMD (III-V), intracellular side |
| C5aR | avacopan | 6C1R | 2.2 | outside 7TMD (III-V), intracellular side |
| Class B | ||||
| GCGR | MK-0893 | 5EE7 | 2.5 | outside 7TMD (VI-VII), intracellular side |
| GCGR | NNC0640 | 5XF1 | 3.19 | outside 7TMD (VI-IV), intracellular side |
| GLP-1R | PF-06372222 | 5VEW | 2.7 | outside 7TMD (V-VII), intracellular side |
| GLP-1R | NNC0640 | 5VEX | 3.0 | outside 7TMD (V-VII), intracellular side |
1. GPR40-TAK-875, GPR40-AP8, GPR40-MK866
GPR40 plays a key role in metabolic homeostasis and can be activated by free fatty acids (medium chain and long chain). GPR40 is highly expressed in pancreatic cells, and GPR40 agonists are an important method in the treatment of type 2 diabetes (T2DM).
A total of two Lipid-facing allosteric sites were found on the GPR40 receptor-lipid bilayer interface.
The targeted small molecules are the local agonist TAK-875 and the full agonist Ago-PAM. We calculated the buried area of TAK-875 receptor and the surface area exposed to the lipid bilayer using PISA web server.
The results show that the surface area of TAK-875 exposed to the lipid bilayer is 286.97Ų, which accounts for 35.1% of the total solvent accessible surface area (SASA) of the ligand.
The buried area of the receptor is 531.74 Ų (64.9%), and the ratio of the exposed area to the buried area is 0.5936. The surface area of AP8 exposed to the lipid bilayer is 177.92 A², accounting for 22.6% of the total area.
The buried surface area of the receptor is 610.74 A² (77.4%), and the ratio of the exposed area to the buried area is 0.2913.
It is worth noting that the ratio of AP8 is smaller than that of TAK-875, which may be due to the different binding modes of the two ligands. The surface area of MK866, which is similar to the allosteric site of TAK-875, is 285.01 A² and 531.58 A², which account for 34.9% and 65.1% of the total SASA, respectively.
The ratio of exposed area to buried area is 0.5362, which is also similar to TAK875.
2. β2AR‒Cmpd-6FA
β2-adrenergic receptor (β2AR) is a typical Class A GPCR, which is widely expressed in vascular and bronchial smooth muscle.
β2AR is activated by endogenous agonists such as adrenaline to mediate cardiovascular function and pulmonary physiological processes, and is an important target for the treatment of vascular and respiratory diseases.
In addition, it is also essential for overcoming immunosuppression and improving the efficacy of immunotherapy.
Recently, a β2AR positive regulator named Cmpd-6FA (a derivative of Cmpd-6) has been identified.
The allosteric site where the ligand binds is located at the receptor-lipid bilayer interface.
The buried surface area of the receptor is 430.71 A², accounting for 49.7% of the total surface area, and 50.3% of the total surface area of the ligand (436.73 A²) is exposed in the lipid bilayer.
3. P2Y1R-BPTU
P2Y1 receptors (P2Y1R) are a subfamily of human purinergic GPCRs that play a regulatory role in thrombosis and induce platelet aggregation.
In addition, it also mediates Ca2+ wave propagation, neuronal axonal initial segment (AIS) and inflammatory response and other physiological processes, and is considered a promising drug target for many diseases.
A new type of antagonist 1-(2-(2-(tert-butyl)phenoxy)-pyridin-3-yl)-3-(4-(trifluoromethoxy)phenyl)urea (BPTU) has Developed for the treatment of thrombosis.
Interestingly, BPTU also showed a unique binding model between the ligand and the allosteric site at the receptor-lipid bilayer interface.
The surface area of BPTU exposed to the lipid bilayer is 265.48 A², which accounts for 39.3% of the total surface area.
The buried surface area of the receptor (409.88 A²) accounts for 60.5% of the total surface area, and the ratio of exposed area to buried area is 0.6477.
4. PAR2-AZ3451
Protease-activated receptor 2 (PAR2) is a self-activating receptor.
PAR2 is highly expressed in many human cells (such as epithelial cells, inflammatory cells and nerve cells) and is associated with a variety of diseases, including metabolic diseases, inflammation and neurodegeneration.
In the past few years, modulating PAR2 function through agonists and antagonists has been considered as a promising therapeutic strategy for modulating specific diseases.
The receptor buried area of the agonist ligand AZ3451 is 460.86 A², which accounts for 59.2% of the total surface area.
The rest of the ligand extends to the lipid bilayer, which accounts for 40.8% (317.74 A²) of the total surface area, and the ratio of exposed area to buried area is 0.6895.
5. CB1 Receptor-ORG27569
Human cannabinoid receptor 1 (CB1 receptor) is most highly expressed in the brain and mediates central nervous system activity caused by cannabinoids.
CB1 receptors play an important role in sleep, memory and pain.
To date, various natural and synthetic cannabinoids and allosteric modulators of the CB1 receptor have been reported for the treatment of various human diseases, such as epilepsy.
Recently, the co-crystal structure of CB1 receptor and its allosteric modulator ORG27569 has been resolved (PDB ID: 6KQI).
The surface area of ORG27569 exposed to the lipid bilayer is 303.33 A², which accounts for 46.2% of the total surface area of the ligand. The buried surface area of the receptor is 353.40 A², which accounts for 53.8% of the surface area.
The ratio of exposed area to buried area is 0.8583, indicating that the lipid The importance of qualitative bilayer to allosteric sites.
6. C5aR-NDT9513727 and C5aR-Avacopan
The human C5a receptor (C5aR), also known as CD88, is highly expressed on immune cells such as T cells, neutrophils, and macrophages.
C5a (74 amino acid anaphylactoxin peptide) binds to the receptor and regulates the release of many effective inflammatory mediators.
The C5a/C5aR axis is involved in neuropathic pain events, and C5aR also acts as a pro-apoptotic receptor in neutrophils.
Studies have shown that C5aR is a potential therapeutic target for a variety of inflammatory diseases, including neuropathic and inflammatory pain, sepsis and cancer.
Recent studies have pointed out that C5aR allosteric non-peptide antagonist is a novel anti-inflammatory treatment method, which helps to solve the problem of C5aR peptide antagonist drugs.
According to reports, the allosteric binding sites of non-peptide antagonists C5aR-avacopan and NDT9513727 are located at the receptor-lipid bilayer interface.
The surface area of NDT9513727 exposed to the lipid bilayer accounted for 38.9% of the total area.
The ratio of the exposed area to the buried area is 0.6380. It is calculated that the surface area of the ligand exposed to the lipid bilayer of avacopan is 222.28 A², the surface area of the receptor buried is 581.26 A², and the ratio is 0.3824.
B. Lipid-Facing allosteric binding sites of class B GPCRs
1. GCGR‒MK-0893 and GCGR‒NNC0640
The G protein coupled glucagon receptor (GCGR) is expressed in a variety of human cells (such as kidney, liver, and pancreatic cells).
The activation of GCGR is triggered by the endogenous peptide agonist glucagon.
Targeting GCGR is an important treatment strategy for diabetes.
Small molecule antagonists against GCGR have been used to treat type 2 diabetes, such as MK0893 (Figure 2) and 4-[1-(4-cyclohexylphenyl)-3-(3). -Methane-sulfonylphenyl))ureidomethyl]-N-(2H-tetrazol-5-yl)benzamide (NNC0640).
The binary complex structure of receptor-ligand shows that the allosteric binding sites of MK-0893 and NNC0640 (PDB ID: 5XF1) are located at the receptor-lipid bilayer interface.
After calculation, the surface area of the ligand buried by the receptor of MK0893 is 450.21 A², which accounts for 51.6% of the total surface area.
The surface area of the ligand exposed to the lipid bilayer is 422.14 A², accounting for 48.4% of the total surface area.
The ratio of exposed area to buried area was 0.9377, indicating the contribution of lipid bilayers to allosteric sites.
The allosteric binding site of NNC0640 is similar to MK-0893.
The surface area of the ligand exposed to the lipid bilayer and buried by the receptor is 403.90 A² and 454.50 A², respectively, and the ratio of the exposed area to the buried area is 0.8887.
2. GLP-1R‒PF-06372222, GLP-1R‒NNC0640 and GLP-1R‒LSN3160440
The glucagon-like peptide (GLP)-1 receptor (GLP-1R) is activated by endogenous GLP-1 peptides.
GLP-1R is widely distributed in human tissues, such as lungs, kidneys, intestines, heart, blood vessels and brain.
The GLP-1/GLP-1R system not only mediates the regulation of insulin release and glucose homeostasis, but also plays an important role in cardiovascular activity, gastric emptying and nervous system.
The binding mode of PF-0637222 and NNC0640 is similar to MK0893.
The surface area of PF-06372222 exposed to the lipid bilayer is 291.93 A², accounting for 36.7% of the total surface area (Table S1), and the receptor buried area is 503.47 A², accounting for 63.3% of the total surface area.
The ratio of the exposed area to the buried area is 0.5798.
The surface area of NNC0640 exposed to the lipid bilayer is 354.68 A², accounting for 40.5% of the total surface area, and the receptor buried area is 521.94 A², accounting for 59.5% of the total surface area.
The ratio of the exposed area to the buried area is 0.6798.
The surface area of LSN3160440 exposed to the lipid bilayer is 236.87 A², accounting for 39.2% of the total surface area, while the buried area of the receptor is 367.93 A², accounting for 60.8% of the total surface area.
The ratio of exposed area to buried area is 0.6438.
The lipid environment regulates the functions of many GPCRs through the interaction between membrane lipids and receptors.
For example, the lipid bilayer can act as a reservoir to gather small molecules around the receptor embedded in the lipid bilayer.
This can increase the duration of the ligand effect by increasing the collision rate between the ligand and the receptor when small molecules are transferred from the solvent to the lipid bilayer.
For example, the molecular dynamics simulation study of the “P2Y1R-BPTU-membrane” system reveals that the lipid bilayer can reduce the conformational space of small molecules and promote the pre-preparation of small molecule binding gestures.
In addition, there are reports that cholesterol, a representative membrane lipid molecule in eukaryotic cells, may play an important regulatory role in the conformational dynamics, ligand binding and oligomerization of GPCRs.
In recent years, several cholesterol-GPCR complex structures (PDB ID 3EML, 5K2D) that have been resolved indicate that cholesterol binds to the allosteric site of GPCR.
The binding site is located at the lipid-bilayer interface or deep TM region.
The effect is mainly mediated by hydrogen bonds between cholesterol groups and TM surface residues.
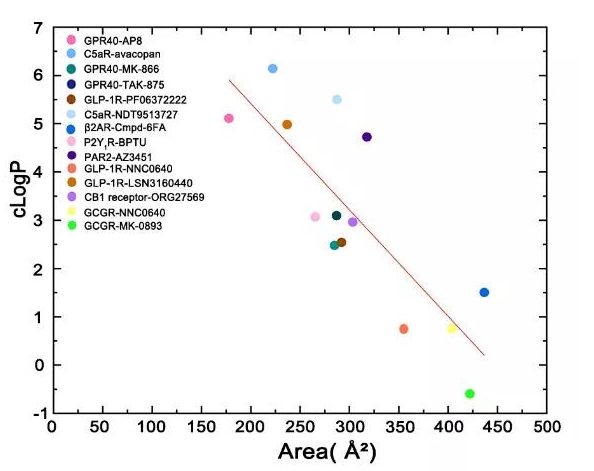
According to the previous summary, when the allosteric modulator binds to the inside of the receptor, the ratio of the exposed area of the ligand to the buried area is mostly less than 0.1, indicating that the surface of the ligand is mainly buried by the receptor.
In contrast, the ratio of the exposed area to the buried area of allosteric ligands targeting Lipid-Facing allosteric sites is mostly higher than 0.5, which indicates that the lipid bilayer is allosteric to the receptor-lipid bilayer. The effect provides an important contribution.
From the perspective of ligand design, these allosteric modulators have two parts, one part is buried under the transmembrane helix, and the other part is exposed in lipids.
The smaller the area of the ligand group exposed to the lipid, the higher the cLogP, which can help people design such regulators in the future to balance the hydrophobicity in protein contact and the size of the exposed part in contact with the lipid bilayer.
Allosteric sites at the GPCR-lipid membrane interface
(source:internet, reference only)
Disclaimer of medicaltrend.org



