Key points for anti-cancer potential of antibody-conjugated drugs
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Key points for anti-cancer potential of antibody-conjugated drugs
Key points for anti-cancer potential of antibody-conjugated drugs. The past decade has been a decade of accumulation and harvest for antibody-conjugated drugs (ADC). After the second new ADC drug Adcetris (Brentuximab vedotin) was approved by the FDA in 2011, 10 ADC drugs have been approved so far.
7 of them have been approved in the past three or four years. Moreover, about 90 candidate ADC therapies in the R&D pipeline are in the clinical development stage, and more than 200 candidate drugs are in the preclinical research stage.
The tissue specificity and cytotoxicity of the new generation ADCs are improved compared with the previous generation products, allowing them to show amazing activity in the treatment of refractory cancers. However, there are still many obstacles preventing the wide application of ADC, including systemic toxicity, insufficient biomarkers for selecting patients, acquired drug resistance, and so on.
Evidence currently available shows that the effectiveness of ADCs is based on complex and subtle interactions between the various components of antibodies, linkers, and cytotoxins, and the tumor and its microenvironment. A few days ago, an in-depth review published by Nature Reviews Clinical Oncology gave a comprehensive description of the mechanisms affecting ADC efficacy and the limitations of ADC drugs. The authors also proposed a variety of strategies to maximize ADC’s anti-cancer potential. Today, WuXi AppTec’s content team will share the highlights of this review with readers.
01 ADC needs cell processing and metabolism to exert its ultimate anti-cancer activity
The traditional description of ADC’s mechanism of action is as follows: The monoclonal antibody part of ADC binds to the target antigen, and is then swallowed into the cell, and then the linker is decomposed, resulting in the release of the load, and the effect of killing cancer cells.
Although this simple description provides the basic framework of ADC’s role, in fact, the process of ADC’s role is more complicated. The author emphasizes that an important point worth considering is that if ADC is to treat tumors, it usually requires tumors to have an effect on ADC.
From this perspective, ADCs can be regarded as a kind of prodrugs, which require the processing and metabolism of target cells to be able to finally exert their full activity. In this review, the author elaborated on the complexity of the steps involved in ADC’s effect.
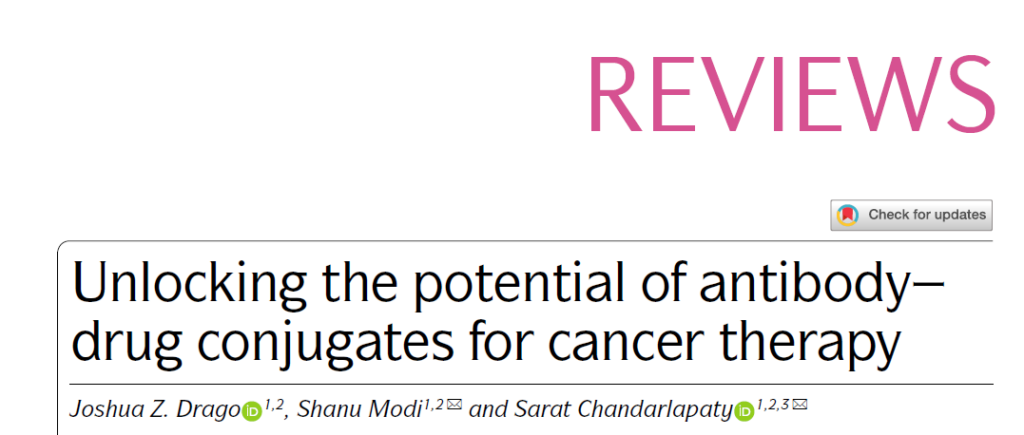
First, due to insufficient purification during the manufacturing process, low linker stability, or metabolism, ADC drug formulations will contain three main components in the blood circulation after entering the human body. The antibody is conjugated to the drug, the antibody without load, and the load without binding to the antibody.
The relative proportions of these three components are very different between different types of ADCs, and as ADC is metabolized in the human body, the in vivo proportions of these three components will also dynamically change, and this feature needs to be established. Pharmacokinetic and pharmacodynamic models to determine the clinical characteristics of ADC.
▲ADC drug is a mixture of three components in the blood circulation (picture source: reference [1])
Compared with traditional cytotoxic drugs, monoclonal antibodies are macromolecules, which means that they have a limited number of penetration into tumors. Current studies have shown that only a small part of the ADC input into the patient can reach tumor cells, which means that It is necessary to consider the intensity of load toxicity when designing ADC.
After the ADC binds to the antigen, the internalization of the ADC-antigen complex is the key to many ADC drug delivery loads. This is usually dependent on the process of antigen-dependent endocytosis (endocytosis) or antigen-independent pinocytosis (pinocytosis).
After internalization, the ADC-antigen complex will be transported to the endosome or lysosome pathway according to the degree of acidification of the organelle. The load connected by the acid-cleavable linker is likely to be released in the early endosome. The design requires a specific protease or the load released by the protein degradation process will be released in the late endosome or lysosome.
Regardless of the load release pathway, some ADCs have the “bystander effect” that can affect surrounding cells, regardless of whether the neighboring cells express the target antigen. For internalized ADCs, it is considered to be an important factor in the tumor-generating activity of ADCs with highly heterogeneous expression of target antigens. Because this effect requires the load to cross the cell membrane, the “bystander effect” requires the cleavable linker to release non-polar load molecules. Polar loads are more likely to remain in the cell.
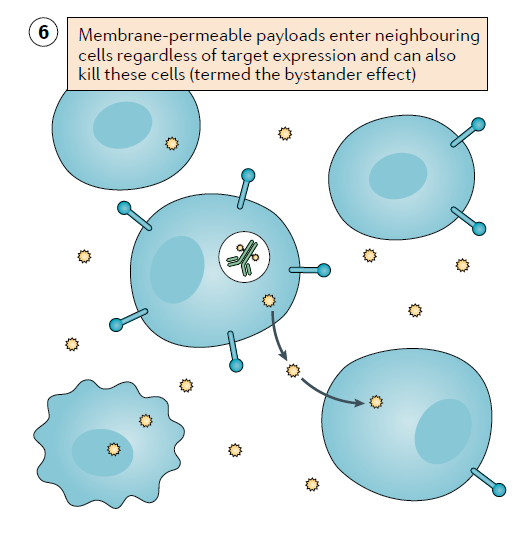
▲Schematic diagram of “bystander effect” (picture source: reference [1])
02 Ways of tumor resistance to ADC
A deep understanding of the emergence of tumor resistance can provide insights into the fundamental mechanism of drug action and promote further drug development. Although the mechanism of resistance to ADC drugs has not yet been fully clarified, the current evidence shows that tumors can evade ADC activity in many ways.
The effect of antigen expression level and patient selection on efficacy
A characteristic of solid tumors is that the expression of target antigens is highly heterogeneous and may be constantly changing. Therefore, selecting the patient population most likely to benefit from ADC therapy requires measuring the level of target antigen expression in tumor tissue. Taking breast cancer as an example, the expression level of HER2 protein may range from almost no expression to high expression due to high-level ERBB2 gene amplification, and the difference in expression level may reach 1,000 to 10,000 times. The current guidelines for testing positive for HER2 include immunohistochemistry (IHC) and fluorescence in situ hybridization. However, whether these tests can predict the efficacy of HER2 targeting ADCs has not been confirmed.
The current results of early studies on the antibody conjugate drug sacituzumab govitecan that target TROP2 show that there is a direct correlation between TROP2 expression levels and patient response. In clinical trials supporting approval, 26% of patients had disease progression, and 37% of patients’ best response was stable disease, meaning that the tumor was resistant to this therapy.
These results indicate that the use of biomarkers to select patients should be an important part of ADC drug development.

Image source: 123RF
The underlying mechanism of ADC acquired resistance
Resistance to tyrosine kinase inhibitors (TKI) usually revolves around escape mutations in the drug target, and ADCs are more complex and diverse due to the complexity of the mechanism of action. The current main drug resistance mechanisms can be divided into three categories, which are reducing the expression level of antigens, changing the intracellular transport pathway, and developing drug resistance to the payload. These potential mechanisms have been verified in preclinical in vitro and animal studies, and clinical evidence to confirm these mechanisms is still limited.
For example, long-term exposure to a breast cancer cell line with a HER2 targeting antibody conjugated drug will reduce the expression of the HER2 receptor, reduce lysosomal acidification and slow down protein degradation. At the same time, they increase the expression of ATP-binding cassette transporter proteins.
Some ATP-binding cassette transporters (such as MDR1, MRP1, and BCRP) have long been considered to play an important role in actively expelling traditional anticancer drugs. The load of some common ADC drugs, such as MMAE, DMA and ozogamicin, can be transported by the ATP-binding cassette transporter, which makes these ADCs more susceptible to this resistance mechanism. However, the load of some ADCs is not easily affected by the ATP binding cassette transporter. For example, Enhertu still exhibits anticancer activity in HER2-positive cancer cell lines with high transporter expression. This may be one of the reasons why it can still show activity in refractory tumors.
03 Strategies to maximize ADC anti-cancer potential
Research in the past few decades has focused the research and development of ADC on the development of ADC drugs that target tumor-associated antigens, carry cleavable linkers and powerful microtubule inhibitors or genotoxic loads. The author predicts that in the next decade, ADC design and clinical applications will usher in more innovations. Strategies to explore the anti-cancer potential of ADC can be divided into R&D strategies for ADC drug design and R&D strategies other than ADC drug design.
R&D strategy for ADC drug design
The ADC itself is composed of three parts: a monoclonal antibody, a linker, and a load, which means that replacing any of these three parts has the potential to increase the effectiveness of the ADC. At present, small-scale drug screening usually uses in vitro or xenograft models to optimize ADC design. They usually compare the effects of combinations of the same monoclonal antibody with different linkers and loads. The review author believes that although this is a rational strategy, it may miss the opportunity to change the pharmacokinetic characteristics of antibodies.
Different antibodies that target the same antigen may have different binding abilities and have very different effects on receptor dimerization and antigen internalization. Current studies have shown that ADC internalization and intracellular delivery pathways have a critical impact on the cytotoxic activity of ADCs. Therefore, monoclonal antibodies optimized for other clinical applications may not be the best choice as the backbone of ADC.
Compared with the wild-type protein, the mutant protein usually has a higher level of ubiquitination and is easier to be internalized and degraded. This means that if ADC is used to target the mutant protein, it may bring a significant clinical response. It is conceivable that targeting ADCs carrying oncogenic mutant proteins (such as certain EGFR mutants) may maximize the tumor specificity of the therapy and reach the level of highly selective TKI.
The progress of bispecific antibody technology has brought more possibilities for innovation. These ADC designs may increase antibody internalization or improve tumor specificity. Current research therapies are already exploring these possibilities. For example, bispecific ADCs targeting different sites on the same antigen can increase receptor aggregation and lead to rapid internalization of the target. In addition, a bispecific ADC targeting HER2 and the lysosomal membrane protein CD63 showed better lysosomal aggregation and load delivery in preclinical experiments.
Another ADC development strategy abandons the traditional monoclonal antibody backbone, but chooses to couple the load to a smaller molecular weight polypeptide fragment or single-chain variable region fragment. The main purpose of these development strategies is to reduce the molecular weight of conjugated drugs, thereby improving the penetration and load delivery of tumor tissues. For example, a drug under development called PEN-221 connects the cytotoxin to the polypeptide chain targeting somatostatin receptor 2. Its molecular weight is only 2 kDa, which is much smaller than the 150 kDa of common IgG molecules. The current technical challenge for such conjugated drugs is that they may be quickly cleared in plasma. However, if they can overcome this obstacle, they can have the potential to treat inaccessible tumors, including poorly innervated tumors and central nervous system tumors.
There are still plenty of opportunities for innovation in load selection. At present, the choice of load is no longer limited to standard cytotoxic drugs, and starts to include targeted drugs and immune drugs. For example, mirzotamab clezutoclax is an ADC targeting B7-H3, and its payload is a BCL-XL inhibitor that promotes apoptosis. It is currently being evaluated in early clinical trials. Other innovative ADC payloads include immunostimulatory factors such as chemokines, Toll-like receptor agonists, or STING agonists. They are designed to target specific tumor-associated antigens to recruit or activate immune effector cells.
R&D strategies beyond ADC drug design
In addition to ADC drug design and pre-clinical research, clinical researchers shoulder the responsibility of exploring the clinical potential of ADC through rational design of clinical trials. This involves two aspects: discovering the patient population most likely to benefit from ADC therapy, and investigating combination therapy options that can have a synergistic effect with ADC therapy, thereby enhancing their clinical efficacy.
For the first task, predictive biomarkers for improved ADC therapy are clearly needed. Currently in clinical trials, immunohistochemistry (IHC) testing is a commonly used method to measure the expression of target proteins. However, IHC is a semi-quantitative test, and there is no clear theoretical basis for how to define what is positive. For different ADC therapies, the IHC signal intensity required to produce anti-cancer activity may vary greatly, and once the threshold is reached, the cytotoxicity may not necessarily increase with the increase of the target antigen expression level.
A variety of factors may affect the therapeutic window and efficacy of ADC, such as target metabolism rate, expression heterogeneity, expression in non-tumor tissues, and characteristics of tumor microenvironment, etc. Therefore, in addition to quantifying target expression levels, the development of other biomarkers that represent tumor sensitivity to ADC will provide important benefits in this area.
In terms of rational development of combination therapies, a number of early clinical trials are currently underway. One of the strategies is to use drugs that target the target antigen to change the dynamic balance of the target antigen, thereby enhancing the sensitivity of cancer cells to ADC. This can be accomplished by stimulating the overexpression of the target antigen or promoting the degradation of the target. For example, the use of irreversible inhibitors against ADC targets (such as the pan-HER inhibitor neratinib in combination with HER2-targeted ADC) can stimulate antigen internalization and ADC endocytosis and activity. Other methods can use feedback mechanisms. For example, inhibiting the MAPK signaling pathway can lead to an increase in AXL expression, thereby increasing the activity of the AXL-targeted antibody conjugated drug enapotamab vedotin in the treatment of melanoma cell lines.
In addition to the use of kinase inhibitors, the combination of ADC with other antibody therapies, such as the combination of ADC with the anti-VEGFA monoclonal antibody bevacizumab, also showed activity in preclinical models. This may be because bevacizumab enhances the delivery efficiency of the drug by changing the vascular innervation of the tumor. Research on the mechanism of tumor resistance may also help discover potential combination therapy targets.
Currently, more than 20 clinical studies are testing the effect of ADC in combination with approved or under-development immunotherapy. The scientific basis of this combination is that ADC-mediated cell death may trigger an immune response and recruit tumor-infiltrating lymphocytes, thereby promoting the recognition of “cold” tumors by immune effector cells.
The author of the article also pointed out that all clinical trials of combination therapies need to consider whether the combination therapy can bring more benefits in the case of potential toxicity. Therefore, the rational design of combination therapy should be based on preclinical data to the greatest extent. Just combining two independently effective drugs does not necessarily bring synergy, but may reduce the therapeutic window due to overlapping toxicity.
04 Conclusion
After decades of research and error correction, technological advancement and a better understanding of the mechanism of ADC activity have brought a variety of ADC therapies that have benefited cancer patients.
The authors emphasize that the rules that apply to the development of standard chemotherapy or antibody therapies do not necessarily apply to predicting the clinical characteristics of ADC. The conceptual model that simplifies ADC into targeted drug delivery may require further improvement to describe the complexity of the ADC’s mechanism of action.
On the whole, after the antigen and antibody are combined, a more detailed understanding of ADC processing and activity will help the development of ADC development field.
If we have a better understanding and utilization of the subtleties of the interaction between ADCs and tumors, it will bring out the true potential of this technology platform and may bring far-reaching and even revolutionary impacts to the treatment of cancer patients.
(source:internet, reference only)
Disclaimer of medicaltrend.org



