Potential Off-Target Effects of mRNA Vaccines: Nobel Prize-Winning Technology Faces Scrutiny
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cambridge Study Reveals Potential Off-Target Effects of mRNA Vaccines: Nobel Prize-Winning Technology Faces Scrutiny
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Cambridge Study Reveals Potential Off-Target Effects of mRNA Vaccines: Nobel Prize-Winning Technology Faces Scrutiny.
The Nobel Prize in Physiology or Medicine for 2023 has been awarded to the pioneers of mRNA technology, Katalin Karikó and Drew Weissman.
Their groundbreaking research, starting in 2005, revealed that modifications to mRNA could significantly reduce unnecessary immune responses, increase stability, and enhance translation efficiency.
This laid the crucial foundation for the development of effective mRNA vaccines against COVID-19.
Their discovery fundamentally altered our understanding of how mRNA interacts with the immune system, making unprecedented contributions to the rapid development of vaccines in the face of significant health threats. Beyond COVID-19, mRNA technology has shown promise in various areas, including cancer, cardiovascular diseases, respiratory, and immune system disorders.
Currently, approved COVID-19 mRNA vaccines contain the N1-methylpseudouridine (m1Ψ) modification, known to reduce the inherent immunogenicity of transcribed mRNA.
However, a recent study by researchers at the University of Cambridge, published in the prestigious journal Nature on December 6, 2023, highlights a potential issue with this modification.
The study reveals that the commonly used N1-methylpseudouridine (m1Ψ) modification in mRNA vaccines can cause ribosomal frameshifting during translation, leading to the production of off-target, unexpected proteins. This phenomenon was observed in individuals who received the COVID-19 mRNA vaccine, and it resulted in unintended immune reactions. The researchers propose optimization strategies for mRNA sequences to minimize potential off-target effects in future mRNA-based vaccines and therapies.
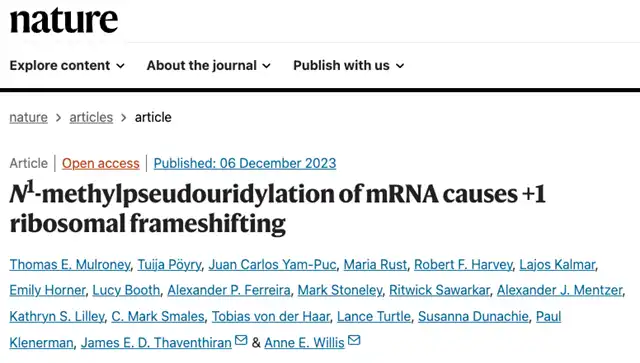
In cells, ribosomes play a crucial role in reading the genetic code of mRNA to synthesize proteins accurately. Any deviation in ribosomal positioning during mRNA decoding can lead to frameshift mutations, altering the protein sequence and potentially triggering immune responses.
The study further analyzed the impact of m1Ψ, 5-methoxyuridine (5moU), and 5-methylcytidine (m5C) modifications on mRNA translation during in vitro transcription.
The findings indicate that the incorporation of N1-methylpseudouridine (m1Ψ) into mRNA can, in some cases (approximately 10%), cause +1 ribosomal frameshifting during translation, resulting in altered proteins outside the target. While most proteins encoded by mRNA were still produced as planned, the study suggests the need to address potential immunogenic side effects.
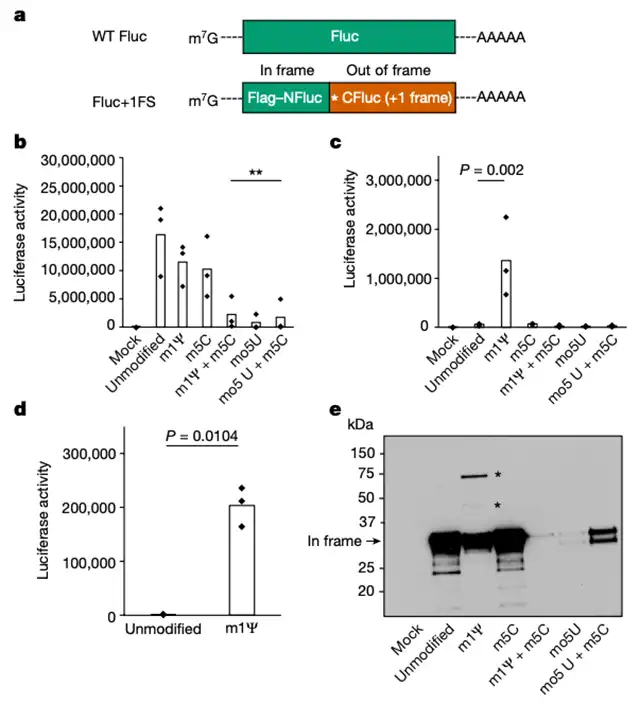
To assess the impact of this frameshifting, the researchers studied the immune response in mice injected with the Pfizer/BioNTech COVID-19 mRNA vaccine (BNT162b2). A comparison with the immune response from individuals vaccinated with the AstraZeneca adenovirus vector COVID-19 vaccine (ChAdOx1 nCoV-19) revealed a higher proportion of immune reactions in the mRNA vaccine group. However, there is currently no evidence indicating adverse outcomes due to these frameshifted products.
The observed +1 ribosomal frameshifting is believed to result from m1Ψ-induced ribosomal stalling during in vitro transcription, leading to frameshifting at the ribosomal slip sequence. Redesigning mRNA sequences to target this slip sequence proved effective in avoiding off-target effects caused by ribosomal frameshifting, ensuring the expected proteins.
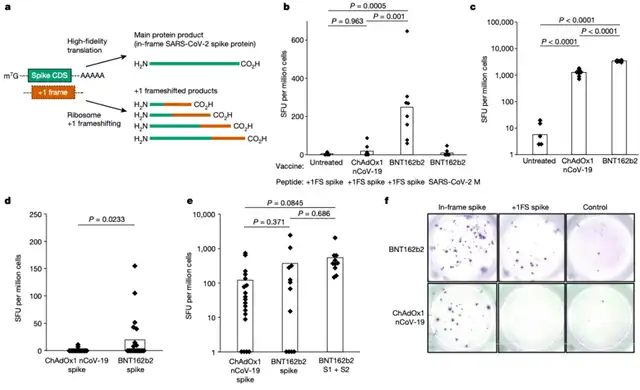
While prior research has demonstrated the safety of COVID-19 mRNA vaccines, this study underscores the need to understand how mRNA treatment design may affect its function. Dr. James Thaventhiran, one of the corresponding authors, emphasizes that this research contributes to our understanding of mRNA therapy design and provides insights into optimizing future mRNA-based therapies to avoid potential translation errors that could reduce efficacy or increase side effects.
For more details, you can refer to the original paper published in Nature: Link to the paper.
Cambridge Study Reveals Potential Off-Target Effects of mRNA Vaccines: Nobel Prize-Winning Technology Faces Scrutiny
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



