Nobel Prize-winning anti-cancer drug: PD-1 inhibitor
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nobel Prize-winning anti-cancer drug: PD-1 inhibitor
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Nobel Prize-winning anti-cancer drug: PD-1 inhibitor. PD-1 inhibitors combined with CAR-T and other new specific tumor immune cell therapy, in hematological tumors, there are preliminary and good data.
Japan won the Nobel Prize, Father of PD-1: Japanese Teacher: Tasuku Honjo
-Japanese Immunotherapy Text JMT-
At 11:30 am on October 1st (5:30 pm on October 1st, Pacific Time), 79-year-old Professor Tasuku Honjo was awarded the 2018 Nobel Prize in Physiology or Medicine.
Professor Tasuku Honjo is a special professor at Kyoto University, researching immunology.
Professor Tasuku Honjo is the 18th scientist to win the Nobel Prize in Japan in 18 years. In 2001, the Japanese government proposed a “Nobel Prize Training Goal” in the Second Basic Science and Technology Plan.
The goal at that time was “30 Nobel Prizes in 50 years.” Overseas public opinion once pointed out: “The Japanese government speaks wildly.” Only 17 years have passed, and 18 have been taken.

Tasuku Honjo, a professor who has been doing research all his life
The 79-year-old professor Tasuku Honjo has never left the campus in his entire life. Whether he is studying in the United States or doing postdoctoral studies at Kyoto University, he stays in the school’s research room to do research. He is making a series of new cell discoveries.
According to Professor Tasuku Honjoyou: “You have to do it until you can’t do it” because of the paranoia and ingenuity in the Japanese bones. The core highland of the world’s cutting-edge medical research, iPS cells, known as “universal cells”, were born in the campus laboratory of Kyoto University.

The “PD-1” gene of Professor Tasuku Honjo was discovered in 1992. The basic principle is: The most immune cells (T cells) in the human body cannot play a role in the combination with cancer cells. This is because there is a blocking substance between the two cells, that is, Professor Tasuku Honjo The discovered “PD-1” gene. As long as the “PD-1” gene is inhibited, the blockage between immune cells and cancer cells can be broken, so that the body’s own immune cells can gradually engulf cancer cells and eventually destroy them.
Professor Rena Changjiang Yamazaki of the Yokohama University of Pharmaceutical Sciences who won the Nobel Prize in Physics (1973) commented on Professor Tasuku Honjo’s major discovery: “It has made a great contribution to the ultimate victory of all mankind against cancer.”
In 2002, Professor Tasuku Honjo’s research results were verified in mice. However, it took 22 years for the results of this research to go from discovery to drug conversion and clinical use. Professor Tasuku Honjo is well aware of the significance of his research results, so after completing the mouse experiment, he is determined to convert his research results into biological drugs to save cancer patients.
As a result, he initially sought out many overseas pharmaceutical companies but received rejection results because the whole society was skeptical about immune cell therapy for cancer.
The “PD-1” gene of Professor Tasuku Honjo was discovered in 1992. The basic principle is: The most immune cells (T cells) in the human body cannot play a role in the combination with cancer cells. This is because there is a blocking substance between the two cells, that is, Professor Tasuku Honjo The discovered “PD-1” gene. As long as the “PD-1” gene is inhibited, the blockage between immune cells and cancer cells can be broken, so that the body’s own immune cells can gradually engulf cancer cells and eventually destroy them.
In the end, a company called “Ono Pharmaceutical Industry” (ONO) extended a cooperative hand to Professor Tasuku Honjo. In September 2014, Nivolumab, the first anti-cancer drug produced by Ono Pharmaceutical Co., Ltd., was approved to be put on the market. This drug can activate the original cells in the body to kill tumor cells with small side effects. The current clinical data shows that some patients with 7 types of malignant tumors such as lung cancer, melanoma, and kidney cancer can achieve complete remission, and the effect is very obvious. According to Ono Pharmaceutical Industry Company, in the past four years, a total of 25,000 cancer patients have used this new anti-cancer drug of Nivolumab. Among them, in 2017, patients who used this new anti-cancer drug: 540 melanoma patients, There were 7,300 lung cancer patients, 2,200 kidney cancer patients, 190 lymphoma patients, 2,400 head and neck cancer patients, and 4,200 gastric cancer patients, for a total of 16,830 patients.
Decryption is the PD-1 inhibitor!
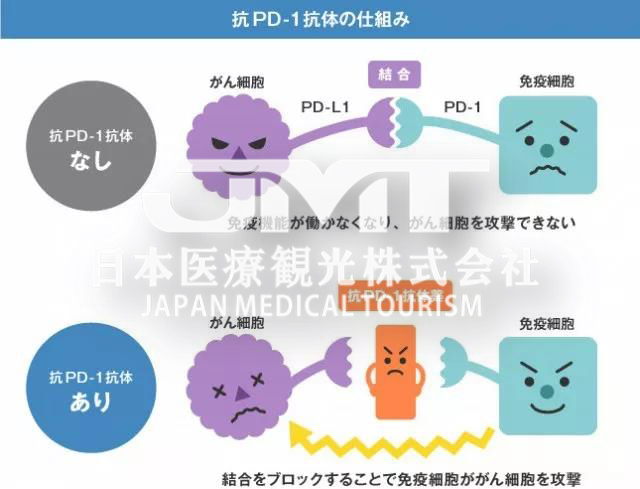
In the past four to five years, he has gained fame in the cancer circle and has been hailed as a rising anti-cancer star; he has even been hailed as a miraculous medicine that can cure cancer by some “patients who don’t know the truth.” This is PD-1, or more accurately, PD-1 inhibitor!
1. What is a PD-1 inhibitor?
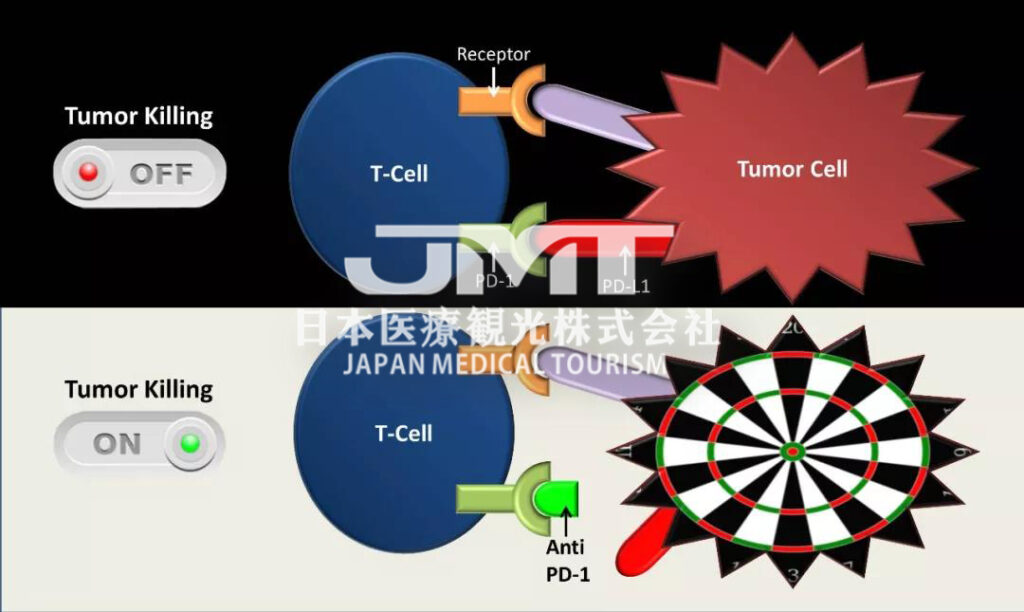
PD-1 inhibitors, including PD-1 antibody and PD-L1 antibody, are a new tumor immunotherapy drug. Unlike surgery, radiotherapy and chemotherapy, and targeted drugs, PD-1 inhibitors themselves cannot directly kill cancer cells, but instead activate the patient’s own immune system to fight cancer.
PD-1 inhibitor, the first clinical trial was started in 2006, and it was officially launched in September 2014. Since then, it has been successively selected by Science and the American Society of Clinical Oncology as the “most progress of the year”, the inventor of PD-1 inhibitor It has also won the Lasker Prize for Medicine, which is known as the “wind vane of the Nobel Prize in Medicine”. The medical community generally predicts that this major invention will eventually win the Nobel Prize in Medicine within the next few years.
2. What are the approved indications for PD-1 inhibitors?
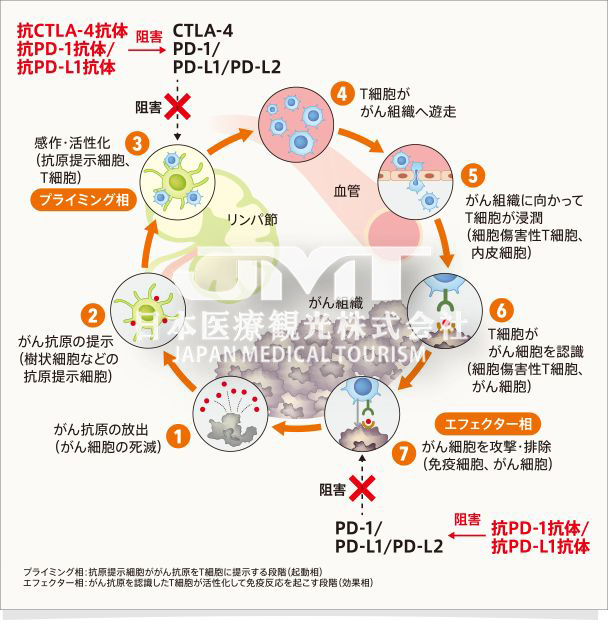
Since September 2014, PD-1 inhibitors have been officially approved by the US FDA for: malignant melanoma, non-small cell lung cancer, liver cancer, gastric cancer, kidney cancer, bladder cancer, head and neck tumors, Hodgkin’s lymphoma , Merkel cell carcinoma and all solid tumors with highly unstable microsatellites (MSI-H).
In addition, PD-1 inhibitors are used in colorectal cancer, esophageal cancer, triple negative breast cancer, nasopharyngeal cancer, ovarian cancer, cervical cancer, prostate cancer, endometrial cancer, glioma, neuroendocrine tumors, and malignant mesothelioma , Non-Hodgkin’s lymphoma and many other solid tumors have shown preliminary and encouraging effects.
3. How to evaluate the efficacy of PD-1 inhibitors?
For most solid tumors (except lymphoma), the average time to onset of PD-1 inhibitors is about 2-4 months. Therefore, after medication, it is the most standard to review imaging every 6-8 weeks.
Imaging shows that the tumor has shrunk or stabilized, and medication can be continued. If the imaging shows that the tumor is enlarged, but the patient can usually take the drug 2-3 times to see the aftereffect, because about 10% of the patients will have “false progress”; if the tumor continues to grow in the second review, then Just consider changing the dressing.
How to improve the cure rate through combination therapy?
Currently, the mainstream partners of PD-1 inhibitors are as follows:
(1) Combined with another immunotherapy drug
PD-1 inhibitors combined with CTLA-4 antibody have been approved for malignant melanoma; Phase III clinical trials have been successful in kidney cancer and non-small cell lung cancer with high TMB. In addition, IDO inhibitors, TIM-3 inhibitors, LAG-3 antibodies and other new immunotherapy drugs are under development.
(2) Combined chemotherapy
PD-1 inhibitor combined with chemotherapy has been approved for the first-line treatment of advanced non-squamous non-small cell lung cancer; similar programs for gastric cancer, bowel cancer, triple-negative breast cancer, etc. also have good preliminary data.
(3) Combined radiotherapy
PD-1 inhibitors combined with radiotherapy have good data in lung cancer, malignant melanoma and other tumors; retrospective studies even suggest that radiotherapy combined with PD-1 inhibitors can increase survival several times.
(4) Combined targeted drugs
PD-1 inhibitors combined with anti-angiogenesis targeted drugs (bevac, axitinib, levatinib, cabozantinib, etc.), there are good preliminary data; but combined with EGFR inhibitors (such as Yi Rui Sa, Tarceva, Chemera, Afatinib, Teresa, etc.), need to be careful, serious side effects may occur.
(5) Combined oncolytic virus
PD-1 inhibitor combined with oncolytic virus T-VEC, in malignant melanoma, the effective rate exceeds 70%, and the complete remission rate exceeds 30%, which is very promising. In other tumors, a variety of oncolytic viruses are under development.
(6) Combined with personalized tumor vaccine
Based on neoantigens produced by tumor gene mutations, peptide or RNA vaccines can be designed and synthesized. PD-1 antibody combined with this kind of private customized and personalized tumor vaccine has preliminary successful experience, which can prevent tumor recurrence and can initially cure advanced tumors in clinical practice.
(7) Combined specific tumor immune cell therapy
PD-1 inhibitors combined with CAR-T and other new specific tumor immune cell therapy, in hematological tumors, there are preliminary and good data.
~~~~~ Nobel Prize-winning anti-cancer drug: PD-1 inhibitor ~~~~~~
(source:chinanet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



