Advancements and Clinical Progress in RNA Therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Advancements and Clinical Progress in RNA Therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Advancements and Clinical Progress in RNA Therapy
The Human Genome Project (HGP) has revealed extensive information about the human genome, significantly enhancing its role in the development of biomedical research.
With advancements in next-generation sequencing technologies, researchers have been able to uncover the role of genetic factors in various diseases such as cancer, rheumatoid arthritis, Parkinson’s disease, and Alzheimer’s disease.
Furthermore, research outcomes have highlighted the crucial roles of coding and non-coding RNAs (ncRNAs), including miRNA, long ncRNAs (lncRNA), circRNA, and siRNAs.
This has laid the foundation for developing potential therapeutic approaches for various diseases by introducing nucleic acids into cells to permanently or temporarily control changes in gene expression.
The development of various delivery systems has addressed the inherent instability of RNA, leading to the emergence of RNA-based therapies involving siRNA, ASO, enzymes, mRNA, aptamers, and CRISPR/Cas, which are now undergoing clinical trials for various diseases.

SiRNA Therapy
SiRNA: SiRNA is produced by the ribonuclease Dicer through a cleavage process, generating double-stranded RNA of 21-25 nucleotides. Once produced, Dicer transfers siRNA to the RNA-induced silencing complex (RISC), which contains Argonaute 2, degrading the target mRNA molecules. SiRNA therapy is being tested in various cancer treatments, such as the siG12D-LODER, a biodegradable polymer matrix containing siRNA targeting KRASG12D, showing promising results in targeting tumors and inhibiting tumor progression.
Polo-like Kinase (PLK): PLK is involved in cell cycle regulation and proliferation, often overexpressed in cancer cells. TKM-080301, a lipid nanoparticle formulation containing synthetic double-stranded siRNA targeting human PLK1 mRNA, has demonstrated good tolerance. While limited anti-tumor effects were observed in advanced liver cancer patients, TKM-080301 showed some tumor inhibition in adrenal cortical carcinoma (ACC).
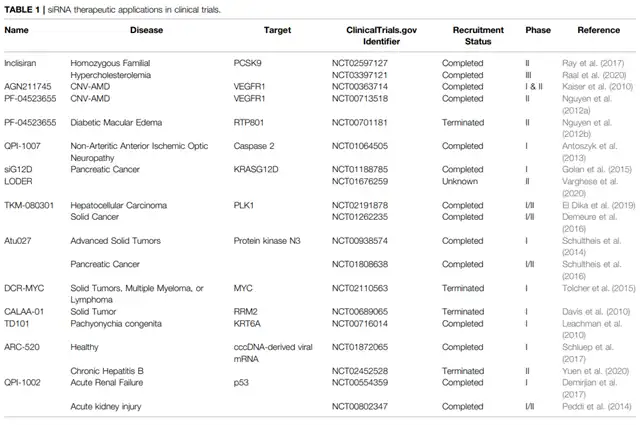
Protein Kinase N3 (PKN3): PKN3, a downstream effector of the phosphoinositide 3-kinase (PI3K) pathway, plays a role in tumor progression and lymph node metastasis. Atu027, a lipid-based PKN3 siRNA formulation, showed good safety in advanced solid tumor patients, with 41% of subjects experiencing disease stabilization. In subsequent studies, Atu027 combined with gemcitabine demonstrated good safety and tolerance in locally advanced or metastatic pancreatic cancer.
Beyond cancer, siRNA therapy holds promise in age-related macular degeneration (CNV-AMD), primary hyperoxaluria (PHs), hepatitis B virus (HBV), liver fibrosis, and idiopathic pulmonary fibrosis.
ASO Therapy
Antisense Oligonucleotides (ASO): ASOs are single-stranded RNA/DNA molecules designed to specifically inhibit mRNA function. ASO therapy has shown promising results in preclinical and clinical trials for various eye diseases. For example, QR-110, a single-stranded, phosphorothioate-modified, 2′-O-methylated splicing-modulating RNA oligonucleotide, targeting CEP290, demonstrated good tolerance and safety in patients with Leber congenital amaurosis (LCA).
Aptamer Therapy
Aptamers: Aptamers, single-stranded nucleic acid molecules binding and inhibiting proteins, are being explored in clinical trials for diseases such as age-related macular degeneration (AMD), diabetic macular edema, and chronic inflammatory conditions.
EYE001, a PEGylated aptamer targeting VEGF, has shown the ability to reduce VEGF-mediated vascular leakage and inhibit retinal neovascularization, leading to significant visual stability or improvement in CNV-AMD patients.
Zimura and pegcetacoplan are PEGylated aptamers targeting complement factors in AMD, demonstrating the reduction of geographic atrophy (GA) secondary to AMD without adverse events.
Enzyme Therapy
Ribozymes: RPI.4610 (Angiozyme), a chemically stable anti-VEGFR-1 ribozyme, has shown good safety, bioavailability, and tumor localization in combination with cisplatin and paclitaxel for advanced solid tumor patients.
OZ1, a tat-vpr specific anti-HIV ribozyme, demonstrated significant increases in CD4+ lymphocytes when delivered via autologous CD34+ cells.
While some successes have been achieved in many clinical trials, challenges such as efficacy and safety concerns have been reported, emphasizing the need for improvement in stability, in vivo activity, co-localization, cell-specific delivery, and sustained stable long-term expression in enzyme-based therapies.
mRNA Therapy
mRNA-Based Therapeutics: mRNA-based therapy represents a novel and efficient drug category, with recent studies highlighting its potential efficacy in treating various malignant tumors and infectious diseases, surpassing traditional vaccine strategies in inducing protective immune responses.
Infectious disease vaccines, particularly mRNA vaccines for COVID-19 developed by Moderna and Pfizer, have demonstrated safety and efficacy in billions of individuals, indicating strong future applications.
For cancer treatment, mRNA vaccines are administered through dendritic cells (DC) either ex vivo-loaded or electroporated, or through direct injection of mRNA with or without carriers. Clinical trials with DC-based mRNA vaccines have shown impressive tumor regression, with some studies indicating persistent tumor growth inhibition.
Direct injection of naked or complexed mRNA is considered a rapid and effective approach. For instance, a Phase I/II trial using intradermal repeated applications of an mRNA vaccine encoding six different tumor-associated antigens (TAA) in metastatic renal cell carcinoma patients demonstrated safety and efficacy, with long-term results showing delayed tumor growth and improved survival rates.
mRNA-4157, an individualized cancer vaccine developed by Moderna, has shown promising results in a Phase I clinical trial, where the majority of patients experienced disease stabilization or responded positively.
CRISPR/Cas9 Therapy
CRISPR/Cas9: The CRISPR/Cas9 system, originally a bacterial defense mechanism, has shown potential for treating genetic diseases such as cystic fibrosis, Duchenne muscular dystrophy, sickle cell anemia, HIV, and β-thalassemia. Studies have successfully used CRISPR/Cas9 to increase utrophin levels in muscles and remove duplicated DMD exons, producing full-length dystrophin in Duchenne muscular dystrophy.
In β-thalassemia, CRISPR/Cas9 has been applied to correct mutations in the human hemoglobin β (HBB) gene, demonstrating effective correction and restoration of HBB expression in red blood cells.
While these studies lay the groundwork for future CRISPR/Cas9 clinical trials, challenges such as off-target effects and ethical considerations need addressing before widespread clinical application.
In summary, RNA-based therapies have emerged as potential intervention strategies for various diseases in recent years. Categorized based on their mechanisms and molecules used, RNA therapies have shown promising results in numerous preclinical and clinical studies. However, challenges, including delivery system improvements, remain, indicating that the era of RNA therapy is on the horizon, with ongoing research and development anticipated in the next decade.
Advancements and Clinical Progress in RNA Therapy
References:
1.RNA Therapeutics – Research and Clinical Advancements. Front Mol Biosci. 2021; 8: 710738.
2. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat RevDrugDiscov. 2021 Aug 25 : 1–22.
3. Clinical and immunological effects of mRNA vaccines inmalignant diseases. Mol Cancer. 2021 Mar 15;20(1):52.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



