T Cell Antigens in Cancer Immunotherapy: A Comprehensive Overview
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
T Cell Antigens in Cancer Immunotherapy: A Comprehensive Overview
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
T Cell Antigens in Cancer Immunotherapy: A Comprehensive Overview
In recent years, significant breakthroughs have been achieved in cancer immunotherapy, bringing substantial clinical benefits.
While various immunotherapy approaches exist, a considerable focus has been placed on cytotoxic T lymphocytes (CTLs) targeting tumor cells.
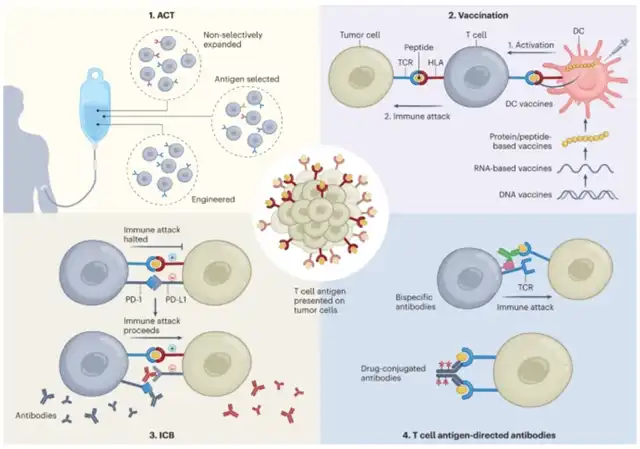
T cells are activated through the interaction of the T cell receptor (TCR) with antigens. V(D)J recombination in the thymus generates a vast diversity of T cell clones, theoretically up to 10^15, each with a unique TCR. Through further selection processes, approximately 10^6-10^10 circulating T cell clones are eventually produced.
T cell antigens are presented on two major histocompatibility complex (MHC) molecules known as human leukocyte antigens (HLAs).
MHC class I molecules are expressed by all nucleated cells, while MHC class II molecules are expressed by antigen-presenting cells (APCs), epithelial cells, and some tumor cells.
The peptides on MHC class I are recognized by CD8+ CTLs, while those on MHC class II are recognized by CD4+ T cells. Cross-presentation of T cell antigens, crucial for CD8+ T cell activation and tumor recognition, involves specific dendritic cell types.
Currently, cancer immunorejection responses are thought to be T cell-mediated, with anti-tumor T cell responses being antigen-specific.
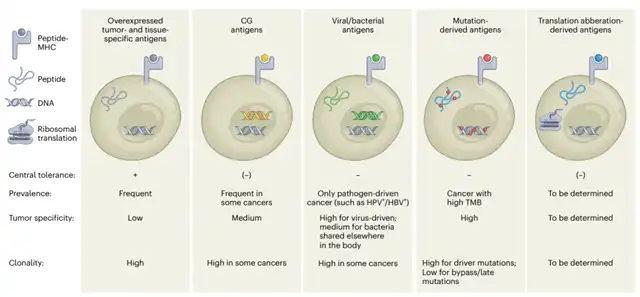
Advances in immunotherapy and available methods for T cell antigen identification have sparked interest in recognizing and characterizing T cell antigens presented by tumors, extending beyond classical tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) to previously less explored sources such as non-standard proteins and bacterial proteins.
-
Self Antigens:
-
Tumor-associated self-antigens exhibit differential expression patterns in tumors without mutations. Examples include MART-1, gp100, and tyrosinase, expressed in melanoma. Tebentafusp, a soluble fusion protein targeting gp100-specific TCR and anti-CD3, has demonstrated clinical benefits in uveal melanoma patients, receiving FDA approval. However, tissue-specific antigens face limitations due to potential damage to surrounding healthy tissues.
-
Cancer germline antigens, originating from proteins expressed only in reproductive tissues, represent another class of self-antigens. Analyzing 153 cancer germline genes revealed their highest aberrant expression in skin, lung, liver, and brain cancers. Their unique expression patterns and high prevalence make them intriguing immunotherapy targets.
-
-
Neoantigens Derived from Genomic Alterations:
-
Features of mutation-derived neoantigens include cancer-related sequence alterations from somatic point mutations, frameshifts, or chromosomal aberrations. Non-synonymous mutations can produce true TSAs, potentially leading to HLA-binding novel peptides. The presence of insertions/deletions and translocations is associated with a significant response to anti-PD-1 or anti-CTLA-4 therapies.
-
Fusion genes, like BCR–ABL in leukemia and EML4–ALK in non-small cell lung cancer (NSCLC), generate T cell-recognizable neoantigens.
-
-
Tumor Antigens from Non-Conventional Transcription and Post-Transcriptional Alterations:
-
Growing evidence suggests frequent non-coding gene translation in tumors, leading to immune responses against tumor antigens from non-coding regions. Various non-coding elements contribute to shared and tumor-specific non-canonical HLA-presented peptides.
-
Examples of antigen-specific T cell responses to intronic sequences include N-acetylglucosaminyltransferase V gene intron, incomplete splice variants of gp100, and 5’UTR region of c-akt oncogene.
-
Translation reprogramming and compromised translation fidelity in cancer cells may produce non-standard translated peptides, potentially serving as new immunogenic antigens.
-
-
Pathogen-Derived Tumor-Associated Antigens:
-
Tumor-associated antigens derived from pathogens are remnants of bacterial or viral infections. Pathogens like Helicobacter pylori, human papillomavirus (HPV), and hepatitis B and C viruses (HBV and HCV) can persist in host cells, mediating malignant transformation.
-
Specific T cell responses against pathogen-derived antigens have shown promise in eliciting immune responses against cancer cells.
-
Emerging evidence suggests the possibility of fungal-derived antigens as another source of tumor antigens.
-
-
Characteristics Facilitating Anti-Tumor Immunity:
-
The effectiveness of any given antigen in anti-tumor immunity depends on a combination of properties. Some, like immunogenicity and effective cross-presentation, are unique to T cell targets, while others, such as population-wide prevalence, disease specificity, clonality, and functional significance, apply to any form of targeted therapy.
-
Optimal combinations of these features are crucial for determining the efficacy of a given antigen. For instance, mutation-derived neoantigens exhibit high immunogenicity but are often personalized, whereas self-antigens are broadly applicable but with lower immunogenicity.
-
In conclusion, understanding the diverse landscape of T cell antigens in cancer immunotherapy is vital for developing effective and targeted treatments.
The exploration of various antigen categories, from self-antigens to neoantigens and beyond, provides a comprehensive perspective on the potential targets for advancing anti-cancer immune responses.
Ongoing research in this field continues to unravel new possibilities and refine strategies for personalized and effective cancer immunotherapies.
T Cell Antigens in Cancer Immunotherapy: A Comprehensive Overview
references:
1.The landscape of T cell antigens for cancer immunotherapy. Nat Cancer. 2023 Jul 6.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



