What Are Major Categories of T-Cell Antigens?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What Are Major Categories of T-Cell Antigens?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What are Major Categories of T-Cell Antigens?
In recent years, significant breakthroughs have been made in cancer immunotherapy, leading to substantial clinical benefits. However, despite the existence of various immunotherapy models, these models largely focus on cytotoxic T cells (CTLs) targeting tumor cells.
T cells are activated through the interaction of specific T-cell receptors (TCRs) with antigens. V(D)J recombination in the thymus generates an enormous diversity of T cell clones, theoretically up to 10^15 clones, each with its unique TCR. Further screening through positive and negative selection processes yields approximately 10^6-10^10 circulating T cell clones.
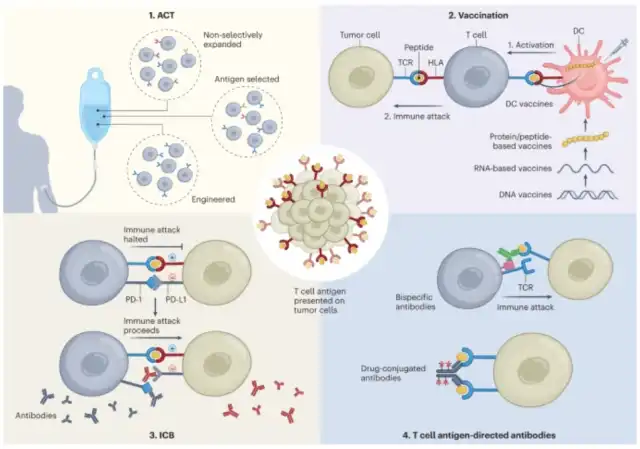
T-cell antigens exist on two major classes of major histocompatibility complex (MHC) molecules known as human leukocyte antigens (HLAs). MHC class I molecules are expressed by all nucleated cells, while MHC class II molecules are expressed by antigen-presenting cells (APCs), epithelial cells, and some tumor cells. Peptides present on MHC-I primarily originate from intracellular proteasomal degradation products and are recognized by CD8+ CTLs, while peptides on MHC-II come from exogenous proteins or membrane proteins degraded by the endosomal/lysosomal system and are recognized by CD4+ T cells. Additionally, there is a process of cross-presentation, where exogenous tumor peptides are primarily presented by XCR1+CD103+ type 1 dendritic cells (DC1s) on MHC-I, and then migrate to tumor-draining lymph nodes, triggering T cell responses against tumor antigens. Cross-presentation is crucial for the maturation and recognition of CTLs by CD8+ T cells.
It is currently widely believed that T cell-mediated antitumor responses are antigen-specific, and advances in immunotherapy and available methods for T cell antigen identification have sparked increased interest in identifying and characterizing T cell antigens presented by tumors. This interest has shifted beyond classical tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) to previously less explored sources of cancer antigens, such as non-standard proteins and bacterial proteins.
Self-Antigens
Tumor-associated self-antigens are non-mutated proteins that exhibit differential expression patterns in tumors. Examples include MART-1, gp100, and tyrosinase, tissue-specific antigens expressed in melanoma. Tebentafusp, a bispecific fusion protein comprising a soluble gp100-specific TCR and an anti-CD3 effector molecule, has demonstrated clinical benefits for uveal melanoma patients and received approval from the U.S. Food and Drug Administration (FDA). It highlights the potential of targeting self-antigens in therapy. However, the use of tissue-specific antigens in treatment is limited by the collateral damage to surrounding healthy tissues due to the similarity in gene expression patterns between tumors and their originating tissues.
Cancer germline antigens represent another category of self-antigens originating from proteins expressed only in germline tissues (fetal testis and ovary) and trophoblast cells. In most healthy tissues, germline genes are epigenetically silenced due to promoter methylation. However, in many human cancers, promoter demethylation reactivates their expression. Analysis of 153 cancer germline genes suggests that they are most frequently aberrantly expressed in skin cancer, lung cancer, liver cancer, and brain cancer. Their unique expression patterns, limited influence by central immune tolerance, and high prevalence in patients make them intriguing immunotherapy targets. However, their expression in tumors is heterogeneous due to changes in DNA methylation status.
New Antigens Derived from Genomic Changes
Mutated-derived new antigens are characterized by cancer-associated sequence alterations encoded by somatic point mutations, frame-shifts, or chromosomal aberrations. Non-synonymous mutations leading to altered proteins can generate true TSAs, and their degradation may result in HLA binding neo-peptides. Single amino acid changes can alter the immunogenicity of HLA-bound peptides, or, if they occur at anchoring positions, can convert non-binding sequences into HLA-binding sequences. Additionally, mutated amino acids can generate new proteasomal cleavage sites, allowing peptide reprocessing and HLA loading.
The advent of next-generation sequencing allows for systematic, comprehensive investigation of mutations in individual tumors. In turn, this data can guide antigen discovery through T cell-based assays or HLA peptidomics. Many new antigens derived from recurrent mutations have been identified, such as CDK4.R24C, KRAS.G12V/C/D, EGFR, and PIK3CA.H1047L.
Less common mutation types, such as insertions/deletions, translocations, and inversions, may also produce new antigens. Analysis of three independent melanoma cohorts found a significant correlation between frame-shift/insertion mutations and responses to anti-PD-1 or anti-CTLA-4. Moreover, analysis suggests that frame-shift mutations create a potentially more effective neo-antigen landscape compared to an equal number of non-synonymous single-nucleotide variations (nsSNVs).
Finally, fusion genes, such as BCR-ABL fusion in leukemia (Philadelphia chromosome) and EML4-ALK fusion in non-small cell lung cancer (NSCLC), have been shown to produce T cell-recognizable neo-antigens.
Tumor Antigens from Non-Canonical Transcription and Post-Transcriptional Aberrations
Increasing evidence suggests that translation of non-coding genes frequently occurs in tumors, and the anti-tumor immune response can target tumor antigens derived from non-coding regions. By combining HLA peptidomics, RNA sequencing, and ribosome profiling data, hundreds of shared and tumor-specific non-canonical HLA-presented peptides from lncRNA, pseudogenes, transposable elements, UTRs, and alternative open reading frames have been discovered.
Examples of antigen-specific T cell responses against intronic sequences include the N-acetylglucosaminyltransferase V gene intron, incomplete splicing intronic regions of gp100, and the 5′ UTR region of the c-akt oncogene. Peptides immunogenic to MHC presentation derived from alternative reading frames include NY-ESO, HER2, telomerase reverse transcriptase, prostate-specific acid phosphatase, and nuORFs with non-AUG translation initiation sites.
Translation reprogramming and reduced translation fidelity in cancer cells can produce non-standard translation peptides, potentially generating new immunogenic antigens. These novel antigens arise from translation disruptions, such as ribosomal frameshifting during amino acid depletion, oxidative stress, or misreading of codons by misregulated tRNAs.
Lastly, post-translational modifications (PTMs) can be deregulated in cancer cells, leading to growth advantages and offering potential targets for cancer immunotherapy. However, whether these PTM-derived antigens can trigger meaningful T cell responses remains to be determined.
Pathogen-Derived Tumor-Associated Antigens
Pathogen-derived TAAs are remnants of bacterial or viral infections. If acute infections are not properly cleared, viruses may persist in host cells and drive malignant transformation. Pathogens directly implicated in cancer include Helicobacter pylori, human papillomavirus (HPV), and hepatitis B and C viruses (HBV and HCV).
Inducing antigen-specific T cell responses against pathogen-derived antigens has become a promising strategy to spark immune responses against cancer cells.
For example, bacterial peptides from different tumors have been found on patients’ HLA molecules, triggering antigen-specific immune responses in melanoma. Antigens originating from other microbial components (such as viral components) might have inherent potential to elicit T cell responses or cross-react with other TAAs in a molecular mimicry fashion. A typical example is the antigen TMP1 encoded by a phage, which activates T cells responsive to PSMB4. Strains of Escherichia coli carrying the phage, abundant in lung and kidney cancers, have been associated with immunotherapy response in human patients.
Another potential source of pathogen-derived antigens is endogenous retroviral elements (ERVs). About 5% of human cancers, especially cervical and oropharyngeal malignancies, involve high-risk HPV strains. ERVE-4, whose expression is associated with the immunotherapy response in clear cell renal cell carcinoma.
Lastly, the recent discovery of fungi in various tumor compositions suggests that fungal-derived antigens may be another category of tumor antigens. Whether they can elicit T cell reactivity remains to be studied.
What are Major Categories of T-Cell Antigens?
Reference:
1. “The landscape of T cell antigens for cancer immunotherapy.” Nat Cancer. 2023 Jul 6.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



