Biogen’s ADUHELM® Faces Market Exit after 970 Days
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Biogen’s ADUHELM® Faces Market Exit after 970 Days: Controversial Journey from FDA Approval to Withdrawal
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Biogen’s ADUHELM® Faces Market Exit after 970 Days: Controversial Journey from FDA Approval to Withdrawal
After being on the market for two years, the world’s first drug to slow the progression of Alzheimer’s disease, ADUHELM®, has been delisted in recent days.
Three years ago, ADUHELM® received approval and entered the market as the first drug designed to delay the progression of Alzheimer’s disease (AD).
However, just two days ago, it exited the stage of new AD drug competition. On January 31, Biogen announced the discontinuation of the development and marketing of the intravenous injection ADUHELM® (aducanumab-avwa) at a concentration of 100mg/mL, along with the termination of the ENVISION clinical trial.
Image Source: Biogen
Biogen stated that the decision to halt the development of Aducanumab is unrelated to any safety or efficacy concerns.
Controversy over 970 days of market presence
On June 7, 2021, the FDA accelerated the approval of Aducanumab for improving the occurrence of Alzheimer’s disease and slowing disease progression. The drug required monthly intravenous infusions and was priced at $56,000 per year.
Aducanumab became the world’s first drug capable of slowing the progression of AD, as opposed to just alleviating dementia symptoms, which was the case with previously approved drugs.
Image Source: FDA
However, controversy surrounded this groundbreaking drug from the moment of its approval. Two Phase 3 clinical trials yielded conflicting results, with unclear clinical benefits.
Biogen submitted clinical trial data to the FDA, including two Phase 3 clinical trials with similar designs initiated in 2015. However, these trials were not fully completed until the application was submitted, with only partial data analysis conducted.
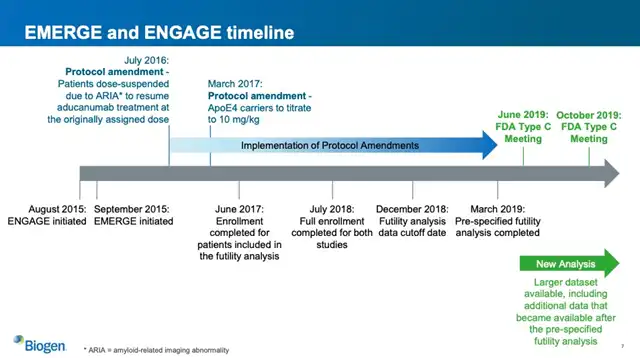
Timeline of the two clinical trials, Image Source: Reference 3
The results of these trials contradicted each other. In one study involving high-dose Aducanumab patients, clinical dementia ratings (CDR-SB scale) showed improvement, while in another study, there was no statistical difference, and the CDR-SB scores appeared worse from a data perspective.
Other results were also discouraging, as multiple cognitive assessment scales showed similar contradictory results: one group showed statistical differences, while another did not. Additionally, there were instances of different trends in changes between the two groups.
Furthermore, Aducanumab was questioned for its unclear clinical benefits. While approved information suggested that Aducanumab could improve cognitive function by reducing Aβ levels in the brains of AD patients, FDA statistical reviews found no evidence linking changes in Aβ levels to cognitive function.
Six months after the accelerated FDA approval, negative reaction reports deepened public concern. A 75-year-old female patient died after receiving Aducanumab treatment, and over 40% of high-dose treated individuals experienced brain edema.
Putting all eggs in one basket: the first FDA fully approved drug in 20 years
Despite doubts about its efficacy, the drug was approved. Some believed the FDA was lenient, and certain clinical doctors stated that they would not prescribe such a drug even if it were FDA-approved.
The FDA persisted in approving Aducanumab, and many regions worldwide did not open their doors to it. In December 2021, the European Medicines Agency refused to grant market authorization for Aducanumab’s use in treating Alzheimer’s disease, questioning the drug’s actual efficacy and requesting Biogen to reevaluate. After careful consideration, in April 2022, Biogen withdrew Aducanumab’s market application in Europe.
Also in April 2022, the Centers for Medicare & Medicaid Services (CMS) in the United States publicly announced the review results: Aducanumab’s Medicare coverage would be limited to clinical trials conducted at medical centers or hospital outpatient departments.

Image Source: Reference 8
Despite ongoing controversy, Biogen did not halt its research in the field of AD drugs. Alongside the announcement of discontinuing the development and marketing of Aducanumab, Biogen emphasized its focus on Lecanemab—the first AD treatment drug fully approved by the FDA in 20 years, listed as one of the top ten scientific breakthroughs in Science 2023.
Based on Phase 3 clinical trial data for Lecanemab, the Lecanemab group showed a relative average decrease of 1.21 points in the primary efficacy endpoint (clinical dementia scale score) after 18 months of treatment, compared to a baseline. In contrast, the placebo group showed a relative average decrease of 1.66 points. The difference of 0.45 points between Lecanemab and placebo was statistically and clinically significant, representing a 27% delay in disease progression.

References: [1,9,10]
Biogen’s ADUHELM® Faces Market Exit after 970 Days: Controversial Journey from FDA Approval to Withdrawal
References:
1、 Biogen to Realign Resources for Alzheimer's Disease Franchise | Biogen
2、 FDA’s Decision to Approve New Treatment for Alzheimer’s Disease | FDA
3、 https://investors.biogen.com/static-files/5a31a1e3-4fbb-4165-921a-f0ccb1d64b65
4、 Alexander GC, Knopman DS, Emerson SS, Ovbiagele B, Kryscio RJ, Perlmutter JS, Kesselheim AS. Revisiting FDA Approval of Aducanumab. N Engl J Med. 2021 Aug 26;385(9):769-771. doi: 10.1056/NEJMp2110468. Epub 2021 Jul 28. PMID: 34320282.
5、 Salloway S, Chalkias S, Barkhof F, et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022;79(1):13–21. doi:10.1001/jamaneurol.2021.4161
6、 https://www.nytimes.com/2021/06/07/health/aduhelm-fda-alzheimers-drug.html
7、 Aduhelm | European Medicines Agency
8、 CMS Finalizes Medicare Coverage Policy for Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease | CMS
9、 https://www.science.org/toc/science/382/6676
10、 van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



