CAR-T therapy: First batch of patients show improvement and stop medication!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CAR-T therapy: First batch of patients show improvement and stop medication!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
CAR-T therapy: First batch of patients show improvement and stop medication!
CAR-T therapy may have the potential to “cure” various immune diseases.
Autoimmune diseases are a class of diseases in which the immune system produces an immune response against the body’s own tissues, with autoantibodies or self-reactive lymphocytes attacking normal cells, leading to tissue damage. Examples include systemic lupus erythematosus, type 1 diabetes, and myasthenia gravis, most of which cannot be cured and require lifelong treatment. The ideal treatment is to preserve protective immunity while eliminating pathologic self-reactive cells.
B lymphocytes/plasma cells are one of the most important effector cells in autoimmune diseases. The chimeric antigen receptor (CAR) T-cell therapy has shown significant efficacy in the treatment of diseases such as acute B-cell lymphoblastic leukemia (B-ALL), B-cell non-Hodgkin lymphoma (B-NHL), and multiple myeloma (MM) by specifically killing B lymphocytes. This suggests a huge potential for CAR-T cell therapy in the treatment of autoimmune diseases. Recently, a landmark clinical study published in The New England Journal of Medicine (NEJM) showed that the “first batch” of 15 severe autoimmune disease patients treated with CAR-T cell therapy maintained remission or significantly reduced symptoms during a median follow-up period of 15 months and had stopped using all immunosuppressive and anti-inflammatory drugs. A concurrent editorial published in NEJM called this a “landmark finding.”
Can CAR-T cell therapy cure autoimmune diseases? Is CAR-T cell therapy effective for all autoimmune diseases? What are the short-term and long-term efficacy and safety of CAR-T cell therapy? “Medical New Perspective” combines recent literature published in top medical journals such as NEJM and The Lancet to help you understand the application of CAR-T cell therapy in autoimmune diseases, for the readers’ enjoyment.
We have compiled high-scoring research related to CAR-T cell therapy from January to February 2024 (covering clinical research, basic medical research, reviews, comments, etc., nearly 50 items). Scan the QR code below to also receive the “2023 Annual White Paper on Frontier Progress of Cell Gene Therapy” produced and compiled by the Content Team of YMKC.
Strategies for CAR Cell Therapy in Autoimmune Diseases
The immune system of patients with autoimmune diseases shows increased reactivity to self-antigens, leading to the destruction of immune tolerance and causing autoimmune responses mediated by T cells and B cells. T cells can cause cytotoxic reactions of effector cells, and B cells can differentiate into plasma cells to produce specific antibodies against self-antigens. Effector cells and antibodies target self-tissues, leading to tissue damage. Meanwhile, modified self-proteins can also induce the production of pathologic autoantibodies, triggering autoimmune diseases. Once this autoimmune cycle is initiated, autoimmune diseases can persist. Conventional treatment only alleviates the autoimmune cycle but does not fundamentally break this cycle.
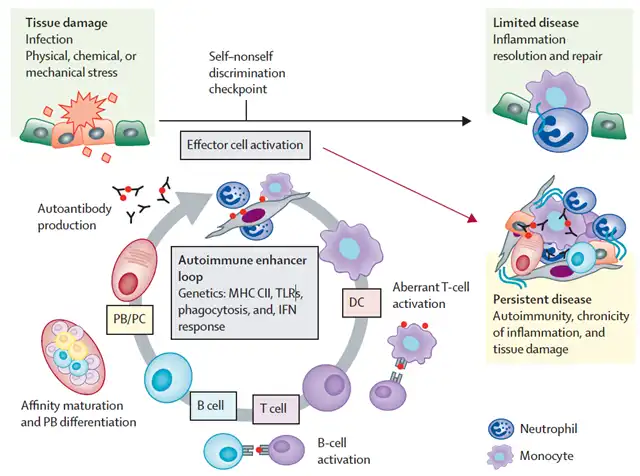
An autoimmune cycle that causes chronic inflammation and tissue damage (Screenshot source: Reference [1])
According to the above immune cycle, potential pathogenic factors of autoimmune diseases may include: 1) the presence of autoantibodies; 2) disease-associated self-reactive lymphocytes; 3) decreased or dysfunctional Treg cells that mediate immune tolerance.
There are three main strategies for CAR cell therapy in autoimmune diseases: CAR-T, chimeric autoantibody receptor T cells (CAAR-T), and chimeric antigen receptor regulatory T cells (CAR-Treg). This article focuses on the progress of CAR-T cell therapy in autoimmune diseases.
Systemic Lupus Erythematosus (SLE)
In patients with systemic lupus erythematosus, the pathological reaction of B cells to DNA and nuclear antigens precedes clinical symptoms. Therefore, clinical practice usually adopts B-cell depletion therapy, immunosuppressive agents, and steroid drugs.
In 2021, the team of rheumatologists and immunologists from Germany led by Georg Schett reported the data of the first severe systemic lupus erythematosus patient treated with CD19-targeted CAR-T cell therapy, finding that 44 days after treatment, the patient’s disease activity index decreased from 16 to 0;
In 2022, they reported the treatment effects of 5 refractory systemic lupus erythematosus patients again, showing that all patients showed improvement, and lupus nephritis was “cured”;
In 2024, the team’s case series study results were published in NEJM. The results showed that 8 systemic lupus erythematosus patients reached the criteria for lupus low disease activity state (LLDAS) and achieved definition of remission of systemic lupus erythematosus (DORIS) after 6 months of treatment. A follow-up of up to 29 months showed that all patients had no disease activity. During the entire observation period, anti-DNA antibodies disappeared and remained negative, complement factor C3 levels returned to normal, and proteinuria disappeared.
According to public information, several CAR cell therapy products for the treatment of systemic lupus erythematosus have made significant progress:
Rijikeolunsi injection (CD19-targeted CAR-T cell therapy) has been granted implied approval for clinical trials for the treatment of moderate to severe refractory systemic lupus erythematosus by the National Medical Products Administration (NMPA) of China;
GC012F injection (CD19/BCMA dual-target autologous FasTCAR-T cell therapy) has been approved for clinical use in China and is intended to be developed for the treatment of refractory systemic lupus erythematosus;
RJMty19 injection (CD19-CAR-DNT cell medicine) has obtained implied clinical trial approval in China and is intended to be developed for the treatment of refractory systemic lupus erythematosus.
…
Idiopathic inflammatory myopathy (IIM)
Idiopathic inflammatory myopathy (IIM) is a group of acquired immune-mediated muscle diseases characterized by inflammatory lesions affecting the skin, muscles, connective tissues, and other organs. Different types of IIM have their own unique manifestations. In 2024, a case series study by the Georg Schett team published in the NEJM showed that three IIM patients achieved significant clinical responses according to the American College of Rheumatology-European League Against Rheumatism (ACR-EULAR) criteria after three months of treatment. Their creatine kinase levels returned to normal, and muscle function improved.
Anti-synthetase syndrome (ASS) is a specific type of IIM, often presenting with myositis, fever, arthritis, and mechanic’s hands. ASS can trigger interstitial lung disease, progress rapidly, and have a high mortality rate, making treatment challenging. The Georg Schett team published a “first-ever” case in The Lancet using CAR-T cell therapy to “cure” ASS. After six months of CAR-T cell therapy, the 41-year-old male patient was “cured,” with complete resolution of muscle lesions, improvement in respiratory symptoms, no longer requiring high-concentration oxygen therapy, and resolution of pneumonia. Surprisingly, even after discontinuing all immunosuppressive drugs, the patient’s symptoms continued to improve. Similarly, the team led by Claudia Lengerke at the University Hospital Tubingen in Germany also brought good news. A case report published in JAMA showed that a patient with anti-synthetase syndrome accompanied by progressive myositis and refractory interstitial lung disease improved rapidly after receiving CD19-targeted CAR-T cell therapy, with significant improvement in clinical symptoms and muscle and lung function.
Neuromyelitis optica spectrum disorders (NMOSD) are immune-mediated central nervous system inflammatory demyelinating diseases, often with acute or subacute onset, rapid progression, and 80% to 90% recurrence rate. The core symptoms include optic neuritis (unilateral, bilateral sequentially or simultaneously decreased visual acuity), acute myelitis, etc. B cells and plasma cells produce anti-aquaporin-4 antibodies (AQP4), which damage astrocytes, leading to autoimmune-related demyelinating changes.
The ability of CAR-T cell therapy to eliminate B cell tumors has provided insights into the treatment of NMOSD. In 2023, Professor Wang Wei’s team at Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology found that using BCMA-specific CAR-T cell therapy to treat 12 patients with AQP4 antibody-associated NMOSD, 11 patients remained disease-free after a median follow-up of 5.5 months.
According to public information, the injection of Ikiosept (BCMA-targeted CAR-T cell therapy) has been approved for clinical use in China for the treatment of NMOSD indications.
Myasthenia gravis (MG) is an autoimmune disease characterized by acquired muscle weakness due to autoantibody-mediated neuromuscular junction transmission disorders. B cells play an important role in the pathogenesis of MG. The MG-001 trial published in The Lancet Neurology showed that after 12 weeks of treatment with Descartes-08 (rCAR-T cell therapy targeting BCMA), 16 adult MG patients showed significant improvements in MG-ADL score, quantitative myasthenia gravis score, comprehensive assessment, and quality of life score. During the 9-month follow-up period, there was a clinically significant reduction in the severity scale of myasthenia gravis. Based on the surprising results of this study, scholars have published related case reports in The Lancet Neurology. A 33-year-old female patient with anti-AchR-positive generalized myasthenia gravis showed improved muscle strength and fatigue after receiving autologous CD19 CAR-T cell therapy for 2 months. The time to extend her arms horizontally increased, and her walking ability also improved without any orthosis.
According to public information, several CAR-T cell therapies for the treatment of MG have made significant progress:
Ikiosept injection (BCMA-targeted CAR-T cell therapy) has been approved for clinical use in China for the treatment of refractory generalized MG;
KYV-101 (autologous CD19-targeted CAR-T cell therapy) has received Fast Track qualification from the U.S. FDA for the treatment of MG and multiple sclerosis;
The future outlook of CAR-T cell therapy
The expected duration of drug-free remission after CAR-T cell therapy for autoimmune diseases still needs further confirmation. Some systemic lupus erythematosus patients have had their disease progression observation period extended to more than 2 years, and most patients are in a state of complete B cell reconstitution during this period. Therefore, some patients with autoimmune diseases may expect to be cured after receiving CAR-T cell therapy. However, this still requires longer follow-up time for observation and confirmation, as it needs to be clarified whether the process of new B cell generation and maturation will be accompanied by auto-reactive B cell clones, autoantibody formation, and thus disease recurrence. If patients do not relapse for many years after treatment, it will strongly promote the clinical application of CAR-T cell therapy as an early intervention strategy for autoimmune diseases.
A commentary published in NEJM on CAR-T cell therapy for autoimmune diseases also points out that the future of CAR-T cell therapy for autoimmune diseases will be influenced by factors such as efficacy, safety, and acceptability. If long-term follow-up can confirm existing results, it will be beneficial for some patients with refractory diseases. If CAR-T cell therapy is truly “tolerant,” there is reason to use it in early and less refractory diseases.
CAR-T therapy: First batch of patients show improvement and stop medication!
References: CAR-T therapy: First batch of patients show improvement and stop medication!
[1]Schett G, Mackensen A, Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023 Nov 25;402(10416):2034-2044. doi: 10.1016/S0140-6736(23)01126-1. Epub 2023 Sep 22. PMID: 37748491.
[2]Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease – A Case Series with Follow-up. N Engl J Med. 2024 Feb 22;390(8):687-700 . doi: 10.1056/NEJMoa2308917. PMID: 38381673.
[3] Luo Xiuna, Ren Jun, Jia Lingyun, et al. Research progress on autoantigens in autoimmune diseases [J]. Chinese Journal of Immunology, 2023, 39(1):172-177.
[4]abian Müller,Sebastian Boeltz,Johannes Knitza,et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Published:February 15, 2023DOI:https://doi.org/10.1016/S0140-6736(23)00023 -5
[5]Pecher AC, Hensen L, Klein R, et al. CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome. JAMA. 2023 Jun 27;329(24):2154-2162. doi: 10.1001 /jama.2023.8753. PMID: 37367976; PMCID: PMC10300719.
[6] Pu Chuanqiang. Idiopathic inflammatory myopathy [J] . Chinese Journal of Neurology, 2019, 52(5): 410-422. DOI: 10.3760/cma.j.issn.1006-7876.2019.05.009.
[7] Wang Weizhi, Wang Huabing. Neuromyelitis optica spectrum diseases [J]. Chinese Journal of Neurology, 2022, 55(5): 511-519. DOI: 10.3760/cma.j.cn113694-20220127-00062.
[8]Qin C, Tian DS, Zhou LQ, et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: phase 1 trial interim results. Signal Transduct Target Ther. 2023 Jan 4;8(1):5. doi: 10.1038/s41392-022-01278-3. PMID: 36596762; PMCID: PMC9810610.
[9]Granit V, Benatar M, Kurtoglu M, et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 2023 Jul;22(7):578-590. doi: 10.1016/S1474-4422(23)00194-1. Erratum in: Lancet Neurol. 2023 Sep;22(9):e10 . Erratum in: Lancet Neurol. 2023 Sep;22(9):e10. PMID: 37353278; PMCID: PMC10416207.
[10]Haghikia A, Hegelmaier T, Wolleschak D, et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 2023 Dec;22(12):1104-1105. doi: 10.1016/S1474-4422(23 )00375-7. PMID: 37977704.
[11] Isaacs JD. CAR T Cells – A New Horizon for Autoimmunity? N Engl J Med. 2024 Feb 22;390(8):758-759. doi: 10.1056/NEJMe2400203. PMID: 38381679.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



