mRNA Therapy Unveils Hidden Cancer Cells: BioNTech’s IL-2 Breakthrough
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
mRNA Therapy Unveils Hidden Cancer Cells: BioNTech’s IL-2 Breakthrough
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
mRNA Therapy Unveils Hidden Cancer Cells: BioNTech’s IL-2 Breakthrough
mRNA Cancer Therapy Scores Big! BioNTech Team Discovers IL-2 mRNA Can Make Invisible Cancer Cells Visible.
Since the rapid development of mRNA technology propelled by the Covid-19 pandemic, there has been anticipation for exploring more applications and modes of mRNA-based therapies in the field of cancer treatment. The potential is certainly not limited to just “personalized therapeutic cancer vaccines.” And now, a breakthrough that refreshes our understanding has arrived.
On March 15, in Cancer Cell, the prestigious journal, BioNTech, the company renowned for developing mRNA vaccines, led by founder Ugur Sahin and colleagues, published their latest research results. They found that using mRNA encoding interleukin-2 (IL-2) as a treatment can make IL-2 a long-acting therapeutic agent, reversing the tumor microenvironment’s status as an “immunological desert” caused by the lack of MHC-I molecule expression by cancer cells, overcoming resistance encountered in many existing anti-cancer treatments.
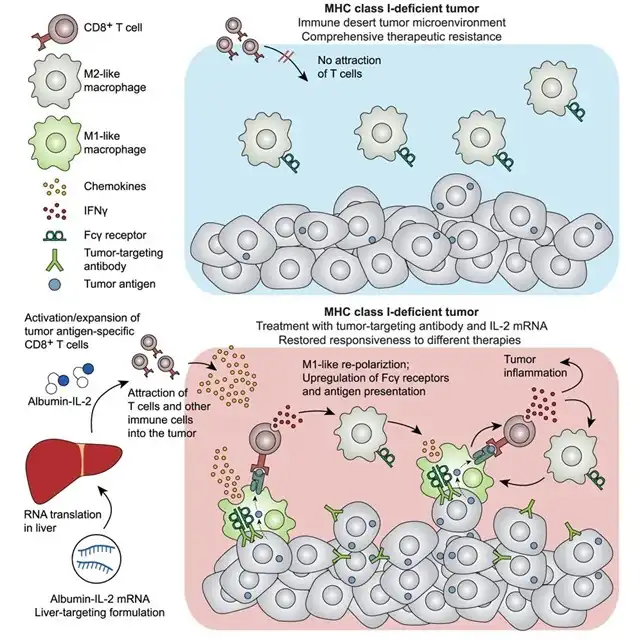
Familiar with immunotherapy, readers may have heard of the concept of “immunological desert” tumors years ago. This desert signifies tumors severely lacking immune cell infiltration, making immunotherapy checkpoint inhibitors ineffective. One of the factors leading to this desert formation is the decrease in antigen presentation due to the lack of MHC-I molecule expression by cancer cells.
Moreover, the impact of the “immunological desert” is not limited to immunotherapy alone. The BioNTech research team simulated the effects of MHC-I molecule expression deficiency by knocking out the B2m gene in cancer cells (B2M encodes B2M, an essential component of all MHC-I molecules) in mice. They found that treatment efficacy with chemotherapy, radiotherapy, and other modalities also significantly decreased in the desert where immune cell infiltration was extremely lacking, even monoclonal antibody-based targeted drugs that have little to do with the mechanism of action and MHC-I molecules were not spared.
So, how can the impact of MHC-I molecule expression deficiency be reversed? Researchers noticed that in the “immunological desert” tumors where B2m gene was knocked out, many cytokines and chemokines were significantly downregulated, including the protagonist of this study—IL-2. Previous studies have shown that using IL-2 or IL-2 pathway agonists can activate NK cells, and NK cells can kill cancer cells without relying on antigen presentation, thus bypassing the barrier of MHC-I molecule expression deficiency.
However, because IL-2 has a short half-life in the body, direct injection of IL-2 therapy can lead to inconveniences and even side effects. This is where BioNTech’s proud mRNA technology comes into play: researchers encapsulated IL-2 mRNA (fused with human serum albumin) using the LPP nanodelivery platform, delivering the mRNA precisely to liver cells where it is translated into IL-2 protein, significantly extending the half-life of IL-2 without any significant adverse reactions. This mRNA-encoded long-acting IL-2 therapy has entered the next stage of experiments.
Researchers in two different MHC-I molecule expression deficient tumor mouse models found that the combination therapy of long-acting IL-2 therapy with monoclonal antibody-based targeted drugs (targeting HER2 and Trp2, respectively) effectively inhibited tumor growth, improved long-term survival rates of mice, and made tumors more sensitive to chemotherapy. Moreover, on the basis of long-acting IL-2 therapy with monoclonal antibody-based targeted drugs, further combination therapy with PD-1 inhibitors or CTLA-4 inhibitors could further overcome the excessive growth of MHC-I deficient cancer cells.
Next, the breakthrough mechanism of long-acting IL-2 therapy needs to be analyzed: whether long-acting IL-2 therapy is used alone or in combination with monoclonal antibody-based targeted drugs, it is enough to change the state of immune cell infiltration significantly lacking in the “immunological desert” microenvironment. The significantly increased infiltrates include CD25+CD8+ T cells, CD25+CD4+ conventional T cells, and KLRG1+ NK cells, among which CD8+ T cells are the most crucial and indispensable for anti-cancer response.
However, researchers found that if macrophages were eliminated from the microenvironment using a CSF1R antibody, the effect of long-acting IL-2 therapy + monoclonal antibody-based targeted drugs combination therapy was basically nullified. These macrophages as a whole polarized towards the beneficial anti-cancer M1 type (M1-TAM), with significantly high levels of co-stimulatory molecule ligands CD80/CD86 expression, indicating they are key to activating CD8+ T cells.
How did macrophages play this crucial role? Further analysis showed that the expression of antigen presentation-related genes in these macrophages (such as H2-Q7, H2-Aa) was significantly upregulated, and CD8+ T cells activated by long-acting IL-2 produced interferon-gamma (IFNγ), which acted on macrophages, causing them to undergo significant reprogramming, acquiring antibody-dependent cell-mediated phagocytosis (ADCP) function, thereby engulfing cancer cells and cross-presenting tumor neoantigens to CD8+ T cells.
Seeing CD8+ T cells recognizing tumor neoantigens, there is no reason for them not to vigorously fight against cancer. We hope that this new treatment modality based on mRNA technology will be translated into clinical practice as quickly as the Covid-19 vaccine.
mRNA Therapy Unveils Hidden Cancer Cells: BioNTech’s IL-2 Breakthrough
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



