Breakthrough: EGFR/HER2 Signaling Key in Pancreatic Cancer Spread
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breakthrough: EGFR/HER2 Signaling Key in Pancreatic Cancer Spread
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Breakthrough: EGFR/HER2 Signaling Key in Pancreatic Cancer Spread
Scientists have made a groundbreaking discovery, revealing that the EGFR/HER2 pathway plays a crucial role in the distant metastasis of pancreatic cancer, often referred to as the “king of cancers”!
It is well-known that EGFR and HER2, both belonging to the epidermal growth factor receptor family, play pivotal roles in the development of cancer. While targeted therapies have revolutionized the treatment of lung and breast cancers, these receptors, EGFR and HER2, continue to play a disruptive role in other cancers through mechanisms that are yet to be fully understood.
Recently, a team from the Li Ka Shing Centre for Cancer Research at the University of Cambridge published their latest research findings in the Cancer Cell journal, unveiling for the first time that pancreatic cancer, a formidable adversary, can accelerate fatal distant metastasis by secreting TGF-β, activating the EGFR/HER2 signals within tumor-associated myofibroblasts (myCAFs). Disrupting this pathway could hold significant value in preventing pancreatic cancer metastasis.
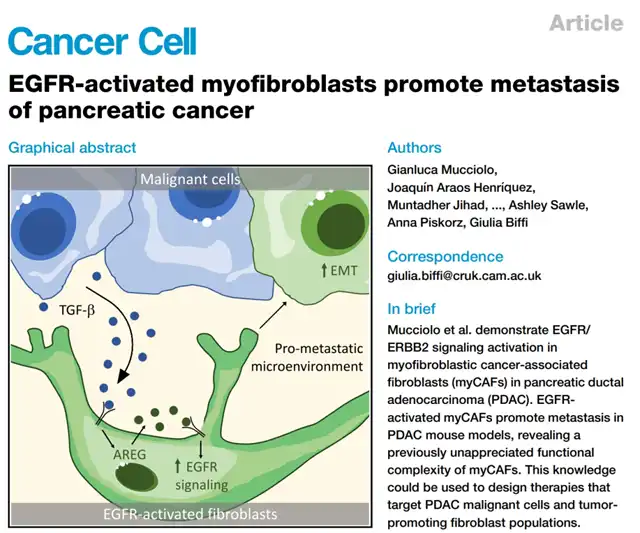
One major characteristic of pancreatic ductal adenocarcinoma (PDAC) is its dense extracellular matrix, which not only makes conventional treatment drugs difficult to penetrate but also provides support for the development of cancer, including various tumor-associated fibroblasts like myCAFs. However, due to the heterogeneity of CAFs, eliminating them entirely could backfire. It is essential to accurately identify pro-cancerous CAFs and intervene precisely.
The Cambridge team’s earlier research identified IL-1 and TGF-β signaling pathways as the major influencers of CAF heterogeneity in PDAC. This current study follows the TGF-β pathway further downstream, seeking downstream signaling pathways and intervention targets. When treating PDAC CAF progenitor cells, known as pancreatic stellate cells, with TGF-β, EGFR and HER2 were the most significantly activated receptor tyrosine kinases (RTKs), both of which must be activated for maximal effect.
Interestingly, treatment with PDAC organ-conditioned medium alone produced similar effects to TGF-β treatment, activating EGFR/HER2 signals in myCAFs. Additionally, single-cell sequencing confirmed that PDAC cells generally express TGF-β. The research team believes that TGF-β from PDAC cells activates myCAFs, and through further experimentation, they confirmed that TGF-β induces a dual-domain protein secretion in myCAFs, crucial for activating the EGFR/HER2 signals (the dual-domain protein being the ligand for EGFR).
So, what are the implications of activating the EGFR/HER2 signals in myCAFs? Firstly, it affects the proliferation of myCAFs themselves. Simultaneously inhibiting EGFR/HER2 signals directly eliminates a subset of myCAFs, characterized by negative CD90 expression. However, solely inhibiting EGFR signals is insufficient, necessitating a combined targeted therapy approach.
After precise elimination of CD90-myCAFs, distant metastases in the lungs and mediastinum significantly decreased in a PDAC mouse model. This indicates that this subset of myCAFs promotes the formation of distant metastases in PDAC. Organoid experiments further confirmed the presence of myCAFs activated by EGFR signals, significantly influencing PDAC’s distant metastases to the lungs, mediastinum, and liver.
Single-cell sequencing revealed that knocking out the EGFR gene in myCAFs significantly reduced the migratory potential of PDAC cells co-cultured with myCAFs, showing a significant downregulation of epithelial-mesenchymal transition (EMT) gene characteristics. Moreover, in solid tumor data from the Cancer Genome Atlas (TCGA), including lung adenocarcinoma, researchers found correlations between the expression levels of TGF-β, dual-domain proteins, and myCAF markers. This suggests that the pro-metastatic role of myCAFs may not be limited to PDAC.
The researchers pointed out that this discovery could explain the synergistic potential of EGFR-TKI class targeted drugs such as erlotinib with immunotherapy. MyCAFs activated by EGFR signals may also promote cancer through other pathways, such as reprogramming their metabolism. Therefore, dual inhibition of EGFR/HER2 signals holds great promise for the future, and further research is imperative.
Breakthrough: EGFR/HER2 Signaling Key in Pancreatic Cancer Spread
References:
[1]Mucciolo G, Henríquez J A, Jihad M, et al. EGFR-activated myofibroblasts promote metastasis of pancreatic cancer[J]. Cancer Cell, 2023.
[2]Özdemir B C, Pentcheva-Hoang T, Carstens J L, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival[J]. Cancer Cell, 2014, 25(6): 719-734.
[3]Biffi G, Oni T E, Spielman B, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma[J]. Cancer Discovery, 2019, 9(2): 282-301.
[4]Li J, Yuan S, Norgard R J, et al. Epigenetic and transcriptional control of the epidermal growth factor receptor regulates the tumor immune microenvironment in pancreatic cancer[J]. Cancer Discovery, 2021, 11(3): 736-753.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



