Pancreatic Cancer: Breakthrough Treatment Doubles Survival Rates
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Pancreatic Cancer: Breakthrough Treatment Doubles Survival Rates
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Pancreatic Cancer: Breakthrough Treatment Doubles Survival Rates
Pancreatic Cancer Risk Soars 16 Times Higher, Linked to This Inflammation! BMJ: This Treatment Doubles Pancreatic Cancer Survival Rates.
Pancreatic cancer, an extremely invasive form of cancer, boasts a mere 12% overall 5-year survival rate for patients.
As a systemic disease, the efficacy of pancreatic cancer treatment is greatly influenced by comprehensive systemic treatment plans.
Recently, The British Medical Journal (BMJ) published a clinical review exploring the latest significant advancements in pancreatic cancer treatment research, offering newfound hope for pancreatic cancer patients.

Risk Factors for Pancreatic Cancer
The review emphasizes that, in addition to factors such as family history, germline mutations/hereditary syndromes, pancreatic cancer is associated with the following lifestyle risk factors:
-
Smoking: Current smokers face an 80% higher risk of pancreatic cancer, while former smokers have a 20% higher risk.
-
Obesity: For every 5-unit increase in body mass index (BMI), the risk of pancreatic cancer increases by 10%.
-
Diabetes: The risk of pancreatic cancer increases by 94% for individuals with diabetes.
-
Chronic Pancreatitis: Patients with chronic pancreatitis have a 16 times higher risk of developing pancreatic cancer.
It’s worth noting that diabetes is also a symptom of pancreatic cancer. Newly diagnosed diabetes in the elderly may potentially serve as an early sign of pancreatic cancer and could be used for early diagnosis. As of now, the correlation between diabetes and pancreatic cancer still requires further research.
Latest Advances in Systemic Treatment for Pancreatic Cancer
The standard treatment protocol for resectable pancreatic cancer involves preoperative surgery followed by adjuvant chemotherapy. In contrast, patients with borderline resectable and locally advanced pancreatic cancer may undergo preoperative treatment (i.e., neoadjuvant treatment) because of the higher likelihood of micro-metastases and lower chances of negative margins. The development of neoadjuvant treatment may help address this challenge.
The treatment approach for metastatic pancreatic cancer usually involves palliative chemotherapy, and a small percentage of patients with excellent chemotherapy responses may consider surgical intervention.
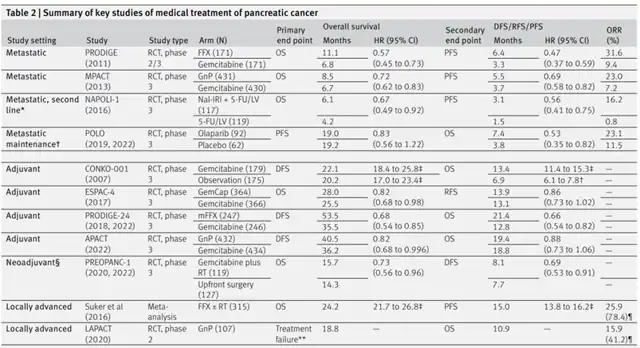
▲ Summary of key clinical trials of systemic treatment of pancreatic cancer (Picture source: Reference [1])
Neoadjuvant Treatment Doubles Pancreatic Cancer Survival Rates
The review emphasizes that 36% of pancreatic cancer patients are unable to undergo postoperative adjuvant chemotherapy after surgical resection, and surgery alone is insufficient for the majority of pancreatic cancer patients to achieve long-term survival. Therefore, preoperative neoadjuvant treatment becomes crucial.
Through neoadjuvant treatment, clinicians can administer higher-dose, more tolerable, and planned systemic treatments to patients before they are weakened by surgery, avoiding treatment delays caused by micro-metastatic lesions (a leading cause of death in pancreatic cancer patients). As of now, two prospective, single-arm, phase 2 studies suggest that gemcitabine plus platinum-based chemotherapy as neoadjuvant treatment has some safety.
The review points out that the PREOPANC-1 trial is the only published analysis of results from a phase 3 trial on neoadjuvant systemic treatment for pancreatic cancer to date. This trial randomized 246 resectable or borderline resectable pancreatic cancer patients to receive neoadjuvant chemoradiotherapy (n=119) or upfront surgery (n=127). The neoadjuvant chemoradiotherapy group received three cycles of gemcitabine plus radiotherapy (36 Gy/15 fr) and four cycles of adjuvant gemcitabine, while the upfront surgery group received six cycles of adjuvant gemcitabine after surgery.
Long-term follow-up analysis results showed that regardless of the resectability of the primary tumor, neoadjuvant treatment provided consistent survival benefits for resectable (HR=0.79) or borderline resectable (HR=0.67) pancreatic cancer patients.
The phase 2 trial ESPAC-5 included 90 patients with borderline resectable pancreatic cancer, randomly assigning them to the neoadjuvant treatment group (n=56) and upfront surgery group (n=33). The treatment strategy for the neoadjuvant group included multi-drug neoadjuvant chemotherapy and single-drug neoadjuvant chemoradiotherapy.
Analysis results showed that compared to patients in the upfront surgery group, patients in the neoadjuvant treatment group had a higher 1-year overall survival rate (1-year overall survival rates for the neoadjuvant treatment group and upfront surgery group were 76% and 39%, respectively; HR=0.29), nearly doubling. However, the sample size of this study was relatively small.
Key Focus on Neoadjuvant Treatment for Pancreatic Cancer
Preoperative neoadjuvant treatment may delay the optimal surgical timing
For resectable pancreatic cancer patients, another point of concern regarding the choice of neoadjuvant treatment is the possibility of disease progression during neoadjuvant treatment, as this may lead to patients missing the opportunity for surgical resection. Results from an analysis of a randomized phase 2 trial (PACT-15) show that compared to postoperative gemcitabine adjuvant chemotherapy or the PEFG regimen (cisplatin, epirubicin, fluorouracil, and gemcitabine) adjuvant chemotherapy, preoperative PEFG regimen neoadjuvant chemotherapy better improves the overall survival of pancreatic cancer patients.
Phase 2/3 trials Prep-02/JSAP-05 randomly assigned resectable or borderline resectable pancreatic cancer patients to a one-month neoadjuvant treatment group with gemcitabine combined with S-1 (n=182) and upfront surgery group (n=180). Both groups received 6 months of S-1 as adjuvant treatment. Analysis results showed a significant improvement in overall survival for patients in the neoadjuvant chemotherapy group (36.7 months vs. 26.6 months; HR=0.72). However, results from the analysis of the phase 2 SWOG S1505 study show that for resectable pancreatic cancer patients, the efficacy of neoadjuvant mFOLFIRINOX regimen is comparable to albumin-bound paclitaxel/gemcitabine treatment for 3 months. Compared to previous adjuvant treatment trials, both groups of patients did not show improved median overall survival (23.2 and 23.6 months).
The optimal treatment duration for neoadjuvant treatment regimens still needs exploration
Recently, a phase 2 trial (NORPACT-1) randomly assigned 140 resectable pancreatic cancer patients to receive FOLFIRINOX regimen neoadjuvant treatment (n=77) and upfront surgery group (n=63). The analysis found that FOLFIRINOX neoadjuvant treatment did not bring more significant survival benefits to patients. However, the analysis results suggest that patients in the neoadjuvant treatment group may not have received sufficient FOLFIRINOX regimen chemotherapy.
The review emphasizes that three large-scale randomized phase 3 trials currently underway may provide important insights into the optimal sequential arrangement and treatment duration of the FOLFIRINOX regimen. Among them, two trials are recruiting patients (ALLIANCE-A021806 trial and PREOPANC-3 trial), and one recently started (NCT05529940).
The first two trials plan to enroll over 300 resectable pancreatic cancer patients to evaluate the survival differences in patients receiving FOLFIRINOX perioperative treatment (8 cycles of neoadjuvant treatment and 4 cycles of adjuvant treatment) compared to those receiving FOLFIRINOX adjuvant treatment (12 cycles).
In addition, the NCT05529940 trial plans to enroll over 600 pancreatic cancer patients to assess the 2-year survival rate differences between patients receiving FOLFIRINOX perioperative treatment (6 cycles of neoadjuvant treatment and 6 cycles of adjuvant treatment) and those receiving FOLFIRINOX adjuvant treatment (12 cycles).
Conclusion
Currently, the incidence of pancreatic cancer is steadily rising worldwide, and this trend is estimated to continue for decades.
Advances in combination therapy based on cytotoxic chemotherapy have played a crucial role in improving the survival rates of pancreatic cancer patients.
In the future, it may be necessary to further explore the practical application value of immunotherapy drugs, radiotherapy, and other treatment methods in certain subtypes of pancreatic cancer patients.
Additionally, there is a need for increased focus on risk assessment, screening, and early detection of pancreatic cancer patients in the future.
Pancreatic Cancer: Breakthrough Treatment Doubles Survival Rates
References
[1] Marco Del Chiaro et al. Advances in the management of pancreatic cancer. THE BMJ. doi: 10.1136/bmj-2022-073995
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



