ADC+IO Combo Doubles UC Survival and Beats Chemo First Time
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
ADC+IO Combo Doubles UC Survival and Beats Chemo First Time
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
ADC+IO Combo Doubles UC Survival and Beats Chemo First Time
On October 22nd, Professor Thomas Powles presented the results of the EV-302 study at the European Society for Medical Oncology (ESMO) Annual Congress and made a significant announcement: “We’ve never before achieved better survival outcomes in first-line treatment of urothelial carcinoma of the urinary tract than with chemotherapy, but now we’ve successfully reached this goal!” The room erupted in thunderous applause, and cheers echoed through the halls.
The EV-302 study revealed that compared to standard chemotherapy, the ADC+IO combination significantly extended Overall Survival (OS) to 31.5 months versus 16.1 months (HR 0.47) and Progression-Free Survival (PFS) to 12.5 months versus 6.3 months (HR 0.45).
This regimen is set to become the new standard of care for first-line treatment of advanced or metastatic urothelial carcinoma.


Background:
Urothelial carcinoma (UC) cells express high levels of the cell adhesion molecule Nectin-4, promoting tumor cell growth. The novel Antibody-Drug Conjugate (ADC) Padcev (enfortumab vedotin, EV) consists of a monoclonal antibody targeting Nectin-4 and a cytotoxic agent (monomethyl auristatin E, a microtubule disruptor), which selectively kills UC cells.
Previously, Padcev’s efficacy and safety in locally advanced or metastatic UC patients who had progressed after platinum-based chemotherapy or PD-1/PD-L1 inhibitors were confirmed in the EV-101 (JCO, 2020), EV-201 (Lancet Oncol, 2021), and EV-301 (NEJM, 2021) studies. Based on these findings, Professor Thomas Powles of Queen Mary University of London led the EV-302/KEYNOTE-A39 study, comparing Padcev in combination with pembrolizumab (EV+P) to standard treatment (chemotherapy) for first-line treatment of advanced or metastatic urothelial carcinoma.
Study Overview:
The EV-302/KEYNOTE-A39 study is a phase III open-label, randomized controlled trial that included 886 previously untreated patients with advanced or metastatic urothelial carcinoma, with a median follow-up of 17.2 months. In this study, patients were randomly assigned to receive ADC in combination with PD-1 inhibitor therapy or standard chemotherapy. The primary endpoints included Progression-Free Survival (PFS) and Overall Survival (OS) assessed based on RECIST 1.1 criteria by a blinded central review (BICR).
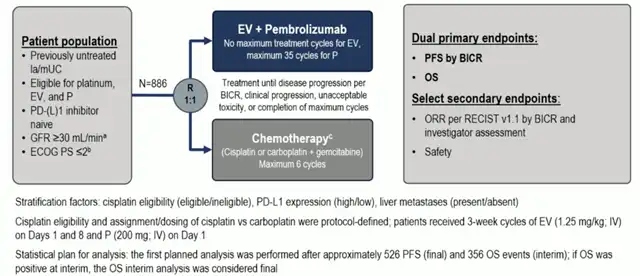
EV-302/KEYNOTE-A39 Study Design
Results:
As of August 8, 2023, the EV+P regimen significantly extended the median PFS for advanced or metastatic UC patients in comparison to standard first-line chemotherapy, reducing the risk of progression or death by 55% (median PFS: EV+P 12.5 months vs. chemotherapy 6.3 months; HR 0.45). Additionally, the ADC combination therapy demonstrated better efficacy in all subgroup analyses compared to standard chemotherapy, irrespective of PD-L1 expression level, primary tumor location, liver metastasis status, ECOG score, or cisplatin eligibility.
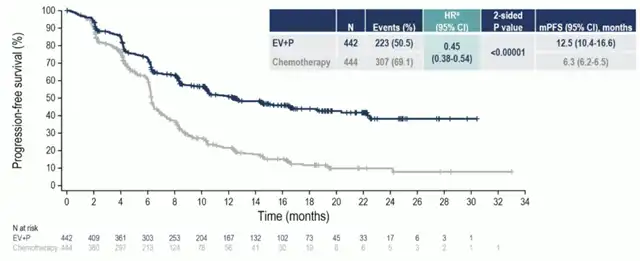
EV-302/KEYNOTE-A39 Study PFS Results
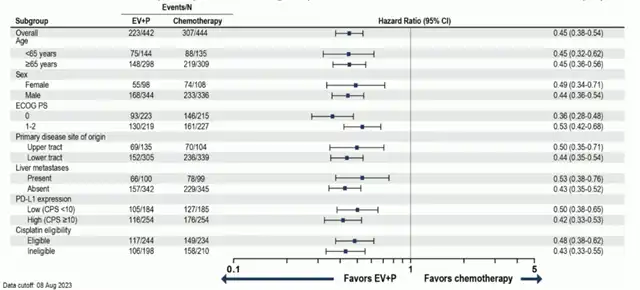
EV-302/KEYNOTE-A39 Study PFS Subgroup Analysis
Regarding the other primary endpoint, Overall Survival (OS), patients in the EV+P group achieved nearly double the OS compared to those receiving standard chemotherapy, reducing the risk of death by 53% (median OS: EV+P 31.5 months vs. chemotherapy 16.1 months; HR 0.47). Similarly, subgroup analyses indicated that the effectiveness of ADC in combination with immunotherapy was not dependent on ECOG score, primary tumor site, liver metastasis status, PD-L1 expression, or cisplatin eligibility.

EV-302/KEYNOTE-A39 Study OS Results
The Objective Response Rate (ORR) in the EV+P group reached an impressive 67.7%, while the chemotherapy group achieved an ORR of 44.4%.
Discussion:
Professor Andrea Apolo pointed out that the EV-302 study’s ORR of 67.7% is similar to what was observed in the EV-103 Cohorts A (ORR = 73.3%) and K (ORR = 64.5%). The EV+P regimen’s median OS, ORR, and Duration of Response (DoR) data represent the best outcomes in first-line clinical studies for advanced or metastatic urothelial carcinoma, surpassing combination therapies such as CheckMate 901, IMvigor 130, and KEYNOTE 361, signifying that this regimen will become the new standard of care for advanced or metastatic urothelial carcinoma.
Safety-wise, the overall rate of Grade 3 or higher adverse events was lower for the ADC combination therapy than for standard chemotherapy (55.9% in the EV+P group compared to 69.5% in the chemotherapy group). Among the most common treatment-related adverse events in the EV+P group were rash (7.7%), hyperglycemia (5.0%), and neutropenia (4.8%). In the chemotherapy group, common adverse events included anemia (31.4%), neutropenia (30.0%), and thrombocytopenia (19.4%). Overall, the safety of the ADC combination therapy was manageable, and no unforeseen adverse events were observed during treatment.
The study also separately examined the treatment-related adverse events of special interest for the ADC drug (EV) and the immunotherapy drug (P). For EV, Grade 3 or higher treatment-related adverse events of special interest included skin reactions (15.5%), peripheral neuropathy (6.8%), and hyperglycemia (6.1%). For P, the rate of severe skin reactions was 11.8%.
Combining efficacy and safety results, the ADC-based combination therapy offers the potential for “increased effectiveness with reduced toxicity” in patients with advanced or metastatic urothelial carcinoma. This study marks a groundbreaking achievement as EV+P outperforms standard chemotherapy in first-line treatment for previously untreated advanced or metastatic urothelial carcinoma, extending patients’ survival by almost twice, all while maintaining manageable safety. The era of first-line treatment for advanced urothelial carcinoma is on the verge of being transformed by ADC in combination with immunotherapy.
Reference:
[1] Powles TB, et al. EV-302/KEYNOTE-A39: Open-label, randomized phase 3 study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). ESMO Congress 2023, LBA6
[2] Rosenberg J, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J Clin Oncol. 2020 Apr 1;38(10):1041-1049.
[3] Yu EY, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm,
ADC+IO Combo Doubles UC Survival and Beats Chemo First Time
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



