Nivolumab: 80% of lung cancer patients survived 5 years after surgery
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nivolumab neoadjuvant therapy: 80% of lung cancer patients survived 5 years after surgery
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nivolumab neoadjuvant therapy: 80% of lung cancer patients survived 5 years after surgery.
When it comes to neoadjuvant lung cancer, we have to mention the well-known Nivolumab CheckMate 816 clinical study.
The Phase III CheckMate 816 study proved that neoadjuvant Nivolumab + chemotherapy compared with chemotherapy can significantly improve resectable NSCLC patients. event-free survival (EFS) rate and pathological complete response (pCR).
Based on the results of this study, nivolumab has been approved by the FDA and domestic NMPA for the neoadjuvant treatment of adult patients with resectable NSCLC from stage IB (tumor ≥ 4 cm) to stage IIIA.
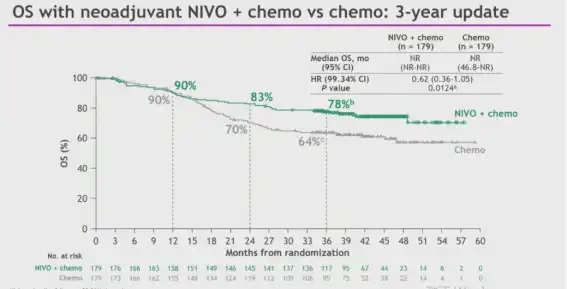
Figure: CheckMate 816 study 3-year follow-up OS results; Source: ELCC meeting in 2023
The 3-year follow-up results of the CheckMate 816 study were released at the European Lung Cancer Conference (ELCC) in 2023.
The median overall survival (OS) of the combination group and the chemotherapy group were not reached, and the 3-year OS rates were 78% and 64%, respectively.
At this year’s AACR meeting, the first Ib/II clinical study of O-drug neoadjuvant therapy for lung cancer announced its 5-year follow-up results.
The clinical study was conducted at Johns Hopkins University and Memorial Sloan Kettering Cancer Center.
The enrolled population was stage IB (tumor ≥ 4 cm) – stage IIIA resectable NSCLC patients received 3 mg before surgery Nivolumab neoadjuvant therapy per kg/2 weeks for 2 cycles, with surgery planned approximately 4 weeks after the first dose.
The primary endpoints of the study are safety and feasibility, and the key secondary and exploratory endpoints are radiological and pathologic responses after treatment, and immune, genomic, and pathologic correlates of hematologic and tumor responses.

Figure A/B is the RFS and OS of the total population, Figure C is the RFS stratified by MPR, and Figure D is the RFS stratified by PD-L1
From August 2015 to October 2016, 20 patients were finally enrolled, all of whom received at least 1 dose of nivolumab.
Postoperative pathological samples from 20 patients were found, 9 patients (45%; 95% CI, 23-68) had significant pathological response (MPR, ≤10% residual viable tumor), 3 patients (10%) had primary tumor A complete pathological response (pCR) occurred.
The 5-year follow-up found that the overall RFS of the patients was 60%, and the overall OS was 80% (including 1 MPR patient who died of traumatic brain injury due to no cancer recurrence).
Of the 9 MPR patients, except 1 patient died of traumatic brain injury without cancer recurrence, 1 patient relapsed, and the remaining 7 patients (RFS 77.7%) achieved cancer-free survival for 5 years.
Both mPR patients achieved 5-year cancer-free survival (RFS 100%).
Six of the nine patients who did not reach the MPR relapsed (RFS 33.3%). PD-L1-TPS score stratification results tend to improve RFS in patients with high PD-L1 expression, and the results of CheckMate-816 have shown that there is a significant association between PD-L1 positivity and pathological response to chemoimmunotherapy and EFS benefit.
Although the original report of this cohort indicated that TMB was predictive of pathological response to neoadjuvant nivolumab, the final results found that TMB stratification was not associated with RFS and OS.
Neoadjuvant immunotherapy for lung cancer has been widely recognized by everyone.
Nivolumab neoadjuvant monotherapy for NSCLC has achieved good long-term clinical outcomes with low incidence of toxicity.
This study further strengthens the confidence that clinicians should use ICB preoperatively.
Further long-term data evaluating the role of neoadjuvant single- and double-agent ICB and neoadjuvant chemoimmunotherapy remain to be seen.
references
[1] Rosner S, Reuss JE, Zahurak M, Zhang J, Zeng Z, Taube J, Anagnostou V, Smith KN, Riemer J, Illei PB, Broderick SR, Jones DR, Topalian SL, Pardoll DM, Brahmer JR, Chaft JE , Forde PM. Five-Year Clinical Outcomes after Neoadjuvant Nivolumab in Resectable Non-Small Cell Lung Cancer. Clin Cancer Res. 2023 Feb 16;29(4):705-710.
[2] Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N EnglJ Med 2022;386:1973–85. [3] FordePM , ChaftJE , SmithKN, AnagnostouV, CottrellTR, HellmannMD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86.
Nivolumab neoadjuvant therapy: 80% of lung cancer patients survived 5 years after surgery
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



