Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- Major Breakthrough in Infertility Research: This Stem Cell Could Be Key to IVF and Other Fertility Treatments
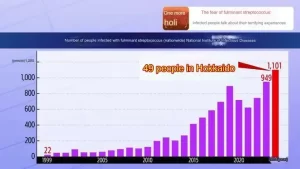
- Flesh-Eating Bacteria Infection Over 1000 Cases in Japan!
- CDC Recommends Updated COVID-19 Vaccines for 2024-2025 Season
- Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- The Critical Role of Immune Cell Triumvirates in Enhancing CD8+ T Cell Function
Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Keto Diet Accelerates Aging and Promotes Cancer Metastasis
The ketogenic diet (Keto Diet) is a high-fat, low-carbohydrate, and appropriately balanced protein and nutrient eating plan. In recent years, the ketogenic diet has become a popular trend, especially among those looking to lose weight, and has been highly regarded in the weight loss community.
When following a ketogenic diet, the body enters a state of “simulated starvation” due to the low intake of carbohydrates, which reduces blood sugar levels. This forces the body to break down fat for energy, leading to rapid fat loss and weight reduction.
In May 2024, researchers from the University of Texas published a study in the journal Science Advances titled “Ketogenic diet induces p53-dependent cellular senescence in multiple organs.” The study showed that the ketogenic diet induces p53-dependent cellular aging in multiple organs. Prolonged adherence to a ketogenic diet can lead to cellular aging in normal tissues, particularly in the heart and kidneys.
However, intermittent ketogenic dieting did not show any pro-inflammatory effects associated with cellular aging, suggesting that intermittent ketogenic dieting might enhance the beneficial effects of the ketogenic diet.
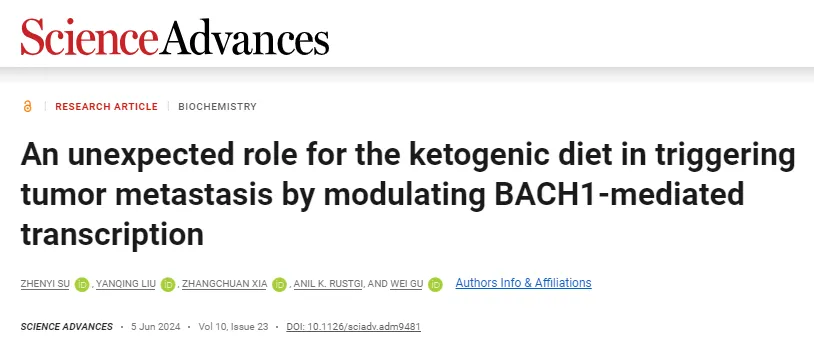
Coincidentally, another study revealed that the ketogenic diet not only accelerates aging but also promotes cancer metastasis. In June 2024, a team led by Wei Gu at Columbia University published a study in Science Advances titled “An unexpected role for the ketogenic diet in triggering tumor metastasis by modulating BACH1-mediated transcription.”
The study demonstrated that while the ketogenic diet can inhibit primary tumor growth in mouse models, it also promotes cancer metastasis. For human cancer patients, the ketogenic diet may pose health risks.
In this study, researchers created a mouse model of breast cancer using a carbohydrate-free ketogenic diet with a fat-to-protein ratio of 4.3:1 to analyze the dual effects of the ketogenic diet on breast cancer.
Analysis of the primary tumors showed that mice on the ketogenic diet had significantly reduced primary tumor growth and lowered blood glucose levels compared to those on a control diet.
After two weeks on the ketogenic diet, the mice’s blood ketone levels significantly increased, indicating effective ketone production and reduced serum insulin levels, potentially decreasing insulin-insulin-like growth factor 1 receptor-mediated tumor growth and progression.
Unexpectedly, while the ketogenic diet inhibited primary tumor growth, it also enhanced tumor metastasis, with the number of metastatic nodules in the lungs significantly increasing in ketogenic diet mice compared to those on a control diet.
The researchers further explored the mechanisms behind the ketogenic diet’s promotion of cancer metastasis. Early studies showed that BACH1 is associated with enhanced tumor metastasis in breast and lung cancers. The research team found that BACH1 promotes tumor metastasis, leading them to analyze the relationship between BACH1 and ketogenic diet-induced cancer metastasis.
Using the CRISPR method, the researchers knocked out the BACH1 gene in the mouse model and found that this significantly eliminated the ketogenic diet-induced pro-metastatic potential, indicating that the ketogenic diet-induced tumor metastasis depends on BACH1.
Further research revealed that under glucose deprivation and ketogenic diet conditions, the expression of the pro-metastatic gene CEMIP was upregulated. CEMIP has been shown to be a key pro-metastatic protein in various tumor types. However, when the BACH1 gene was knocked out, glucose deprivation and the ketogenic diet did not upregulate CEMIP expression.
Activating Transcription Factor 4 (ATF4) is a stress response transcription factor that plays a key role in cellular responses to various stress conditions. Researchers found that during glucose deprivation, ATF4 protein levels significantly increased and specifically bound to BACH1, leading to the recruitment of ATF4 to pro-metastatic target promoters, enhancing BACH1-mediated transcriptional activation, and ultimately promoting tumor metastasis.
In the mouse model, just three weeks on the ketogenic diet led to a significant increase in the expression levels of BACH1’s pro-metastatic targets in the breast cancer model. The ketogenic diet also increased CEMIP protein levels and enhanced BACH1-ATF4 interaction.
Finally, analysis using the GEPIA2 database developed by Peking University found that elevated BACH1 and CEMIP expression levels are negatively correlated with patient survival rates, indicating that BACH1 and CEMIP expression have important clinical significance in breast cancer progression.
This suggests that similar mechanisms may exist in humans. For human cancer patients, the ketogenic diet may pose health risks and should be approached with caution.
In conclusion, this study demonstrates that while the ketogenic diet can inhibit primary tumor growth, it also promotes cancer metastasis and reveals the mechanism behind this effect. Inhibiting the BACH1 gene can block ketogenic diet-induced cancer metastasis.
Paper Links:
- https://www.science.org/doi/10.1126/sciadv.ado1463
- https://www.science.org/doi/10.1126/sciadv.adm9481
Keto Diet Accelerates Aging and Promotes Cancer Metastasis
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.