Coronary heart disease treatment: Methylnaltrexone | Morphine | Ticagrelor
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Coronary heart disease treatment: Methylnaltrexone | Morphine | Ticagrelor
Coronary heart disease treatment: Methylnaltrexone | Morphine | Ticagrelor. The peripheral opioid receptor antagonist methylnaltrexone can only mildly affect the plasma levels of ticagrelor and its metabolites, but cannot increase the absorption rate of ticagrelor, nor can it accelerate its inhibitory effect on platelets. Therefore, the drug does not yet have sufficient clinical application value.
JACC: Cardiovascular Interventions

Oral P2Y12 receptor antagonists are the key to preventing thrombotic complications in patients with acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI). Recent studies have shown that patients with ST-segment elevation myocardial infarction have delayed onset of action after oral P2Y12 receptor inhibitors (such as ticagrelor), and the full antiplatelet effect is not achieved until 4 hours after the medication. Studies have determined that morphine and The use of other opioids (such as fentanyl) is the cause of these problems.
Recently, in an article published in JACC: Cardiovascular Interventions, a journal of Elsevier, Francesco Franchi MD and other scholars discussed whether intravenous methylnaltrexone can offset the pharmacokinetics and pharmacodynamics of ticagrelor by morphine. The impact of learning was explored and evaluated.
1 Research plan
Patients with stable coronary artery disease (n=30) receiving aspirin were randomized to receive methylnaltrexone (0.3 mg/kg intravenously) or the corresponding placebo. After the administration of methylnaltrexone or placebo, all patients received intravenous morphine injections (5 mg). After 15 minutes, 180 mg of ticagrelor was given. Pharmacokinetics (PK) and pharmacodynamics (PD) were evaluated at 12 time points.
The two groups of patients were cross-tested after a 7±2 day drug washout period.
PK analysis includes the determination of plasma levels of ticagrelor and its main active metabolite (AR-C124910XX). PD evaluation includes VerifyNow P2Y12 (a platelet function analyzer), platelet translucent aggregation method, and vasodilator-stimulated phosphoprotein.
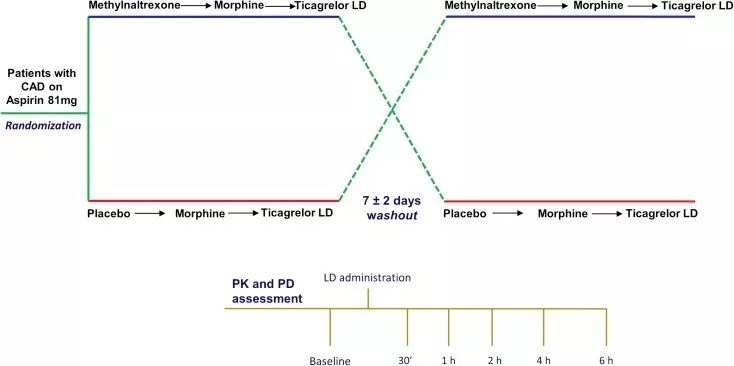
CAD = coronary artery disease; LD = loading dose; PD = pharmacodynamic; PK = pharmacokinetic
Schematic diagram of research plan design
2 Research results
Only slight changes in plasma ticagrelor and its main active metabolite levels were observed after administration of ticagrelor. In patients treated with methylnaltrexone, the maximum plasma concentration and the area under the plasma concentration-time curve during the period from 0 h to the last test were 38% and 30% higher than those of patients receiving placebo, but reached the highest plasma concentration. No difference was observed at the time point of concentration. There was no significant difference in PD index at each time point.
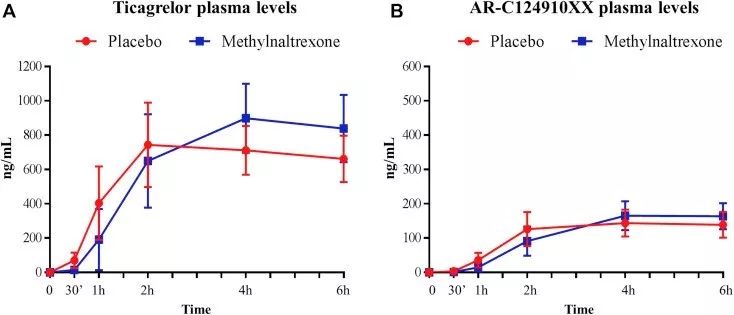
Changes in the pharmacokinetics of ticagrelor and its main active metabolites after taking methylnaltrexone or placebo
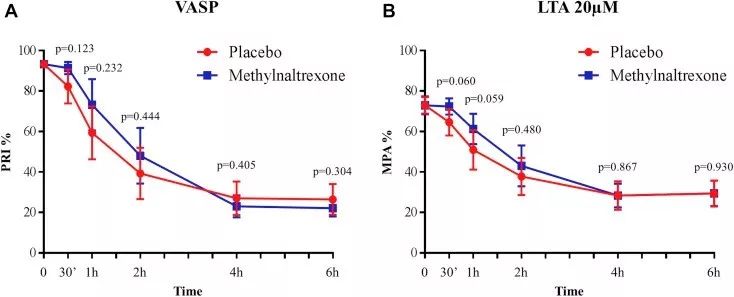
After taking methylnaltrexone or placebo, use vasodilators to stimulate phosphoprotein and light transmittance aggregation for pharmacodynamic evaluation
(A) Platelet reactivity index measured with vasodilator-stimulated phosphoprotein (VASP)
(B) Using 20 μmol/L adenosine diphosphate as an agonist, the maximum platelet aggregation measured by the light transmission aggregation method (LTA)
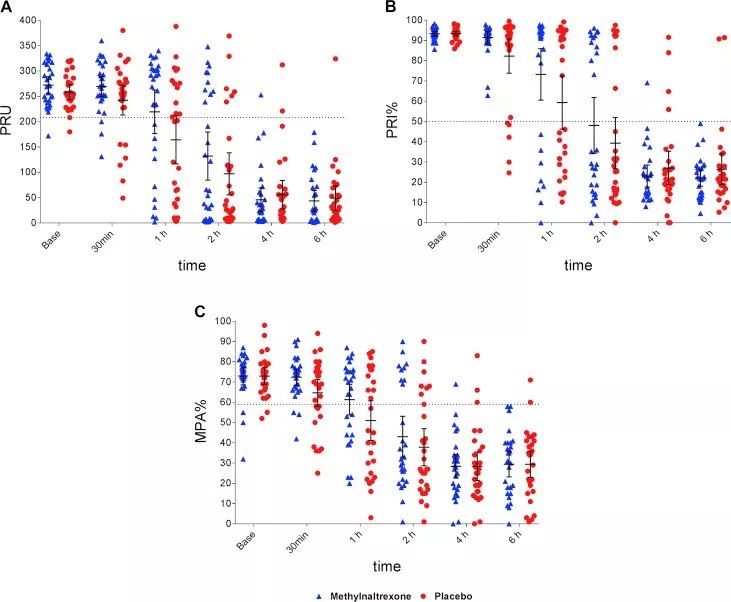
After taking methylnaltrexone or placebo, platelet function, reactivity index, and maximum aggregation.
3 Research conclusion
The peripheral opioid receptor antagonist methylnaltrexone can only mildly affect the plasma levels of ticagrelor and its metabolites, but cannot increase the absorption rate of ticagrelor, nor can it accelerate its inhibitory effect on platelets. Therefore, the drug does not yet have sufficient clinical application value.
(source:internet, reference only)
Disclaimer of medicaltrend.org



