mRNA nano-drugs combined with PD-1 significantly improve cancer treatment
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
mRNA nano-drugs combined with PD-1 significantly improve cancer treatment
mRNA nano-drugs combined with PD-1 significantly improve cancer treatment. Science sub-issue: mRNA nano-drugs combined with PD-1, significantly improve the effect of cancer treatment.
On March 25, 2011, the US FDA approved the first immune checkpoint blocking drug-Ipilimumab (trade name: Yervoy) for the treatment of advanced melanoma.
Since then, more and more immune checkpoint inhibitors have been approved for the market and have shown good results in a variety of cancer treatments.
However, only about 30% of cancer patients can benefit from immune checkpoint blocking therapy, and other patients respond poorly to immune checkpoint blocking therapy.
PTEN gene is a classic tumor suppressor gene, and its mutations are present in many kinds of cancers. Clinical studies in recent years have shown that PTEN is directly involved in tumor immune regulation.
However, it is not clear whether the restoration of functional PTEN can regulate the immunosuppressive tumor microenvironment (TME) and improve the sensitivity of tumors to immune checkpoint inhibitor therapy.
Recently, Professor Jinjun Shi of Harvard Medical School, Researcher Wang Hao of China National Nanoscience Center, Professor Mei Lin of Sun Yat-sen University School of Pharmacy, etc., as co-corresponding authors, published the title: “Reactivation of the tumor suppressor PTEN” in the journal Science Translational Medicine, a subsidiary of Science. by mRNA nanoparticles enhances antitumor immunity in preclinical models” research paper. The revised paper was also selected as the cover paper of the current issue.
This research combines mRNA and nanotechnology to construct an mRNA nanomedicine that encodes PTEN protein. The nanomedicine can efficiently deliver exogenous PTEN mRNA to the tumor site and successfully restore the tumor suppressor function of PTEN, and induce the immunogenic death of tumor cells.
The mRNA nanomedicine has shown excellent therapeutic effects and good safety in different tumor models (melanoma xenograft tumor, prostate tumor in situ tumor and transgenic mouse model). The PTEN mRNA nanomedicine successfully repaired tumor loss Or the mutant PTEN protein functions and realizes the reversal of the tumor immunosuppressive microenvironment, improves the response of tumor cells to PD-1 monoclonal antibody, and improves the effect of anti-tumor immunotherapy.
This study shows that mRNA nano-medicine repairing tumor suppressor genes can increase the sensitivity of tumors to immune checkpoint inhibitor therapy and provide new ideas for the treatment of malignant tumors.

Messenger RNA (mRNA) has recently shown strong application prospects in the field of biomedicine, such as protein replacement, gene editing, and vaccine development. Unlike plasmid DNA, mRNA does not require a nuclear envelope for effective transfection, so the chance of integration into the host genome is negligible. Compared with DNA therapy, mRNA also provides more consistent and predictable protein expression kinetics.
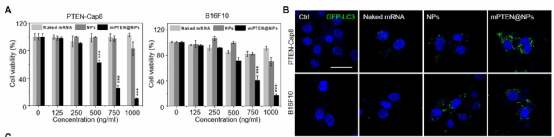
The research team first prepared a new polymeric nanoparticle platform composed of mPEG-PLGA and cationic lipid material G0-C14. The mPEG-PLGA copolymer can be used as a carrier to self-assemble into nanoparticles, and cationic G0-C14 is used with PTEN mRNA is complexed, and then PTEN mRNA is encapsulated in the core of mPEG-PLGA nanoparticles (mPTEN@NPs).
Experimental results show that nanoparticles can protect mRNA from ribonuclease degradation, and effectively introduce mRNA into the cytoplasm of tumor cells, thereby restoring the expression of PTEN.
Studies have reported that the restoration of PTEN expression can inhibit the growth of human cancer cells. Therefore, the research team first wanted to determine whether the use of mPTEN@NPs can also reduce the survival rate of mouse cancer cells. They found that the introduction of human mPTEN@NPs restored autophagy and immunogenic cell death (ICD) induced by PTEN. Further experiments have shown that mPTEN@NPs triggers ICD at least in part through autophagy-mediated pathways.
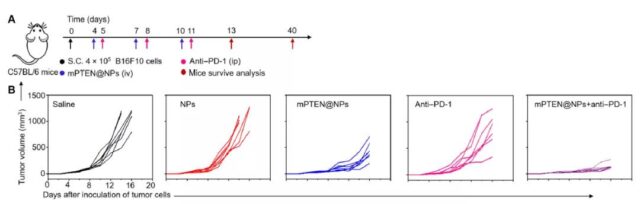
Next, the research team further explored whether PTEN restoration can activate the anti-tumor immune response in the body. They constructed a mouse model of melanoma and treated it with mPTEN@NPs to evaluate the anti-tumor immune response. They found that PTEN can effectively induce the release of autophagy and injury-related molecular patterns in vivo through mPTEN@NPs, trigger anti-tumor immune activation, and reverse the immunosuppressive tumor microenvironment.
Previous studies have shown that PTEN mutations cause adverse reactions to immune checkpoint blocking therapy in cancer patients such as melanoma. Therefore, the research team further investigated whether PTEN’s restoration of anti-tumor immunity can improve the therapeutic effect of immune checkpoint inhibitors in PTEN mutant melanoma models. The experimental results show that in the PTEN mutant melanoma model, mPTEN@NPs combined with anti-PD-1 monoclonal antibody can significantly improve the tumor treatment effect.
The above results show that mPTEN@NPs can not only effectively trigger the anti-tumor immune response, but also improve the effect of anti-PD-1 monoclonal antibody in the treatment of PTEN mutant tumors.
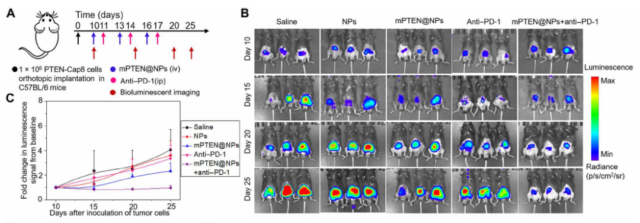
Finally, the research team further evaluated this combination treatment strategy for the treatment of prostate cancer, which usually responds poorly to immune checkpoint blocking therapy or is inherently resistant. The research team constructed an orthotopic prostate cancer mouse model and performed three cycles of mPTEN@NPs and anti-PD-1 monoclonal antibody combined therapy. The experimental results showed that mPTEN@NPs significantly promoted tumor immunogenic cell death (ICD), induced immune memory, and restored the tumor’s sensitivity to anti-PD-1 monoclonal antibodies.
Overall, the research developed a polymer nanoparticle platform for delivery of PTEN mRNA to tumor cells. mPTEN@NPs successfully triggers the anti-tumor immune response by inducing the activation and release of autophagy. In addition, PTEN reactivation reduces the immunosuppressive tumor environment and improves the sensitivity of PTEN-deficient or mutant tumors to immune checkpoint blocking therapies.
This study suggests that mRNA nanomedicine combined with immune checkpoint inhibitors can activate anti-tumor immune responses and improve tumor response to immune checkpoint blocking therapy, which has a strong application prospect
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



