Why does U.S. give Booster Shot to people over 65 but not everyone?
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Why does U.S. give booster shot to people over 65 but not everyone?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why does U.S. give booster shot to people over 65, but not everyone?
Not only the United States began to implement booster shot, Europe and China have also begun to recommend booster shot to high-risk individuals after two doses of COVID-19 vaccine.
Faced with less than two years of understanding of the COVID-19 virus and rapidly changing epidemic, many times our decision-making has to be based on relatively incomplete information, and the same is true for booster shot.
This encourages us to improve relevant research as soon as possible, supplement the completeness of relevant data, and at the same time remind ourselves to be cautious in making decisions based on missing information.
Many developed countries in Europe and the United States have begun to formulate and even implement the COVID-19 vaccine booster shot vaccination plan for the general population.
However, for the COVID-19 vaccine that only started to go on the market at the beginning of this year, another shot will be given to “enhance” in just a few months. The scientific nature and necessity of this is still a very controversial topic.
The US Food and Drug Administration (FDA) external expert meeting on Pfizer/BioNTech vaccine booster shot on September 17 may be the first open and transparent debate on the significance of booster shot and the scientific nature of related policies.
In more than eight hours of analysis and discussion, the Centers for Disease Control and Prevention (CDC), the FDA and Pfizer/BioNTech each listed information, and even Israel, which was the first to implement booster shot, sent health officials and researchers to provide information about the country. The latest data.
In the end, the expert meeting rejected Pfizer/BioNTech’s booster shot proposal by an absolute majority, and instead recommended that booster shot be provided to the elderly over 65 and other high-risk groups.
This program is now officially approved by the FDA and recommended by the CDC.
This meeting and the CDC recommended booster shot meeting on September 22-23, provided a large amount of existing data and decision-making basis discussion on booster shot, which not only allows many people who pay attention to booster shot to understand the current situation of booster shot, but also deserves all formulations.
The booster shot policy is even a reference for the management department of the general epidemic prevention policy.
Principle: Is it a booster shot or a third dose?
In various reports, a benefit often mentioned about booster shot is that it can greatly increase antibodies. This is also the key data used by pharmaceutical companies such as Pfizer/BioNTech to apply for the listing of booster shot.
A substantial increase in antibodies was detected after booster shot vaccination, reflecting the immune response stimulated by booster shot. But the immune response is far more than the antibody part, and the scientific principle of booster shot is not limited to the increase of antibodies.
On September 17, the US FDA expert meeting and the CDC’s multiple discussion meetings on booster shot all mentioned that from the perspective of principle, if the COVID-19 mRNA vaccine vaccinators are vaccinated with the third dose, it is a matter of changing the initial vaccination procedure from two injections to Three shots, or booster shot other than the first vaccination?
Both are third doses. Is there a difference in the initial vaccination program?
In fact, the number of injections for different vaccines is different. For example, the shingles vaccine is two shots, while the hepatitis B vaccine is three shots. This difference in the number of shots is based on the perfection of the immune response.
For vaccines, the first shot will stimulate the initial immune response. This immune response involves the activation of B cells to produce specific antibodies against the antigen introduced by the vaccine and the formation of some memory B cells.
These memory cells do not produce antibodies, but they retain the memory of the antigen. When they encounter the same antigen again, they can quickly replicate and differentiate into a large number of B cells that can produce antibodies.
Generally speaking, the immune response stimulated by the first shot of the vaccine is not strong, as the antibodies produced are not many and the formation of memory cells is also limited.
After a period of time after the first shot of vaccination-at least until the immune response induced by the first shot drops, the body’s immune system can produce a stronger immune response, the so-called boost, by vaccinating the second shot. In one process, a large number of antibodies will be produced, and antibodies with stronger binding capacity or more diverse recognition will appear.
At the same time, memory cells will be further improved, and some B cells can also differentiate into plasma cells. Plasma cells are very long-lived, they will migrate to the bone marrow and produce antibodies for a long time, allowing the body to obtain long-term immune protection.
Due to the rules of immune response during vaccination, most vaccines require two injections, and there must be a certain interval between the two injections. However, not all vaccines can get the most complete immune protection after two injections. This is why many vaccines such as hepatitis B vaccine have to take the third dose to complete the vaccination.
Going back to the new coronavirus vaccine, as a newly developed vaccine, such as Pfizer/BioNTech’s mRNA vaccine, it is now known that very good immune protection can be obtained after two injections. But is the immune response induced by two injections the limit of the human immune system?
This is unknown.
If the immune response induced by the first two shots is already at the limit of the human immune response that this type of vaccine can stimulate, then the third dose only stimulates immune memory and again produces a large number of antibodies, but includes memory cells, the degree of diversity of antibodies, etc.
There will be no improvement. The human body does not continuously produce large amounts of antibodies that it does not need, so the high antibodies stimulated by the third dose will also decrease over time. In other words, the effect of this booster shot is only to temporarily increase the antibody titer in the body.
If the results of the first two doses of mRNA vaccination are not the limit of human immune response, the introduction of the third dose may be like the third dose of hepatitis B vaccine, which can further improve immune protection.
This may have a variety of manifestations, such as stimulating the formation of more memory cells, so that the next time you encounter a virus, you will have a faster and stronger immune response and better protection; another example is the production of more “mature” antibodies, which are Virus binding ability is stronger or recognition is more diversified, enhancing protection against mutations; or forming more long-acting plasma cells, which slows down the curve of antibody decline, allowing the body to be protected for a longer period of time .
If the third dose mRNA vaccine can indeed play a role in further improving the immune response, and it is even inferred that the initial vaccination process of the COVID-19 vaccine should have been three injections, then the vaccine should be designed as a three-dose vaccine instead of a two-dose vaccine.
Unfortunately, there is not enough data to point out whether booster shot can only temporarily increase the antibody titer or can improve the entire immune response. Pfizer/BioNTech only provided the antibody titer one month after the third dose inoculation at the FDA expert meeting.
Although this titer is three times the peak after the second injection, it is impossible to distinguish whether the third dose improves the overall immune response of the vaccinated person or raises the antibody for a short period of time.
Some recent studies on mRNA vaccines have shown that after the second injection, the diversity of antibodies, memory cells, and cellular immunity seems to have reached a limit [1, 2].
If these studies are universal, then the significance of booster shot will be limited to short-term increases in antibodies.
However, these studies are still at an early stage, and the results of different groups of people may be different. For example, the elderly may have a weak immune response, even if the first two injections of the general population can reach the limit of the immune response, for the elderly or people with underlying diseases But not necessarily.
But in any case, booster shot still needs more complete research, not limited to the antibody titer at a time cut-off point, to clarify the role of booster shot.
Timing: Do you need a booster shot now?
The scientific principle of booster shot also affects another important question: when is booster shot needed, or is booster shot needed now?
If booster shot only temporarily increases antibodies, then the timing of use will depend on when the antibodies need to be increased.
Many booster shot plans now use 6 months as the limit. The main basis for this division is that in tracking the antibody titers of some vaccines, it is found that after 6 months of vaccination, the antibody in the body has dropped a lot from the peak. Published 6-month tracking data of neutralizing antibodies [3].
It was found that half a year later, the serum of the vaccinated person could still neutralize multiple mutant strains, including Delta, but the titers of neutralizing antibodies were higher than those just after vaccination. The time has dropped significantly, that is, more serum is needed to neutralize the same amount of virus in the experiment.
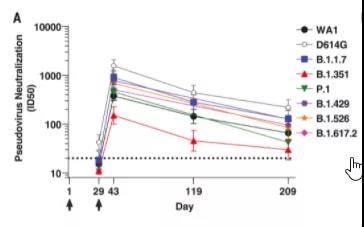 6 months after Moderna vaccination, there are still neutralizing antibodies in the body, but the titer is significantly lower than the peak time | Source [3]
6 months after Moderna vaccination, there are still neutralizing antibodies in the body, but the titer is significantly lower than the peak time | Source [3]
Studies have shown that higher neutralizing antibody titers correspond to better COVID-19 vaccine effectiveness. Simply from the perspective of increasing the body’s antibodies, it seems that there is no problem in increasing the antibody titer by playing booster shot because of the decline in the body’s antibodies after half a year.
But this approach lacks the most fundamental basis, that is, scientists have not yet clarified the lower limit of antibodies necessary for vaccine protection.
That is to say, although the antibody titer did drop significantly after half a year (this is also expected, because the human body does not have to produce a large amount of an unusable antibody without encountering the virus), but it cannot be said that the vaccine at this time The protective effect has fallen sharply.
Still from Moderna’s analysis, according to its Phase III clinical trial, it was found that even if the population with no neutralizing antibody detected after vaccination, the vaccine’s effectiveness in the next three months is still 50%, and the neutralizing antibody titer is 100 In the population with a neutralizing antibody titer of 1000, the effectiveness is 90% and 96%, respectively [4].
Therefore, it is not only the decline in the neutralizing antibody titer that it is not clear whether the vaccine fails and needs to be enhanced. Even if the booster shot greatly increases the antibody, how much vaccine protection can correspond to the increase needs to be reviewed.
After all, the 10-fold difference in the neutralizing antibody is for The first two doses of Moderna vaccine only brought a 6% difference in effectiveness. For example, Pfizer/BioNTech’s booster shot increased the antibody titer to 3 times the peak after the second dose.
The change in vaccine effectiveness is still unknown. It may not change as much as the antibody titer.
In addition, if the increase in antibodies is the benchmark, the antibodies will also decrease after the peak after hitting the booster shot. A natural question is whether you will frequently hit the booster shot to maintain the high level of antibodies in the future.
At the FDA expert meeting, some experts from the United States asked Israeli health officials whether they plan to use booster shot again later if the increase in effectiveness is only a temporary increase in antibodies.
Earlier, an Israeli official said that it needed to prepare a fourth injection [5], but at the expert meeting, Israeli health officials stated that they had no plans to do so.
If the third dose is to perfect the immune response, then the question should be how often the third dose can be used to perfect the immune response. How long is this time, is it 6 months? It is not clear now. In theory, the longer the interval, the better the effect of this perfect immune protection enhancement.
But if it proves that it can be done at shorter intervals, then it is entirely possible to complete the three-shot vaccination at a shorter interval to improve the vaccination effect as soon as possible.
Some scientists have also put forward another idea, that is, whether it is possible to improve the immune protection of the vaccine by changing the interval between the first two shots of the vaccine. At the FDA expert meeting, someone suggested that the current COVID-19 vaccination procedures are very radical.
For example, Pfizer/BioNTech uses three-week intervals between the two injections. Is it because such short intervals lead to insufficient immune response and maintain the effectiveness of the vaccine. not enough?
If the interval between two injections is extended, can the immune protection be more effective and avoid the need for booster shot in the future?
However, extending the interval between the two injections will make the vaccinated person stay in the half-vaccinated state for a longer period of time, without adequate protection, and may not be better in terms of risk and benefit. It is even more meaningless for people who have been vaccinated because they have already been vaccinated. People can’t change the interval between the previous two stitches.
In short, in terms of scientific principles, the main evidence for the third dose or booster shot now is that antibodies can be greatly increased. However, in addition to antibodies, it is unknown whether the overall immune response is perfect, how often the third dose is better, how effective it can be, and how long it can last.
In this case, another way of discussing the necessity of booster shot is based on the effectiveness of vaccines, especially the actual changes in the effectiveness of protection against critical illnesses. Absolute prevention of infection itself is a very high requirement.
Especially nowadays, vaccines have to deal with the highly contagious Delta mutant strain. The infection rate in many countries in Europe and the United States is very high, which is equivalent to that the vaccinators have been exposed to a large amount of virus for a long time. In a high environment, the vaccination rate is not ideal in many countries due to various reasons.
These factors are superimposed, and it is not realistic to expect vaccines to completely block infection or spread. Therefore, maintaining the protection of vaccines against severe illness is a more reasonable and more critical goal.
Various studies have shown that the role of mRNA vaccines in protecting against infections or mild illnesses has declined to a certain extent, but the protection of severe illnesses is still maintained at a relatively high level.
Among them, the decrease in protection against mild cases may have the effect of both the time of vaccination and the effect of Delta. But even in this regard, the decline may be limited. Pfizer, at the request of the FDA, compared the infection rate of the population with a median vaccination time of 9.8 months and 4.7 months in a phase III clinical trial.
People who have been vaccinated for a short period of time are indeed at a lower risk of infection, but when converted to effectiveness, the difference is actually quite limited-if the effectiveness of the person who was vaccinated for 4.7 months is 86%, the effectiveness of the person who was vaccinated for 9.8 months is 80% [6 ].
Many studies on the protection of critical illnesses have shown no significant decline. For example, a study by Pfizer and Kaiser found that the effectiveness of the vaccine against infection dropped to about 60% after 4 months of vaccination, but the protection of critical illnesses did not change at any age. [6].
On September 22, Moderna published the final analysis of the Phase III clinical trial in the New England Journal of Medicine, and found that in the trial with an average follow-up time of 5.3 months, the effectiveness of preventing severe illness was 98% [7].
These studies all point to the fact that the protection of the mRNA vaccine against severe illness may last for a very long time. Obviously, it cannot support the urgent necessity of booster shot.
The strongest evidence for booster shot comes from Israel. An Israeli study published in the New England Journal of Medicine before the FDA expert meeting showed that 12 days after booster shot vaccination, the risk of infection for people over 60 years old decreased by 10 times, and the risk of severe illness also decreased similarly [8].
However, it should be noted that this study was followed up for less than two weeks after 12 days of inoculation with booster shot. This brings up the question of how long the validity can be maintained. At the same time, health officials in Israel stated that 60% of critically ill patients in the country are those who have received two doses of the vaccine.
For them, it is necessary for them to improve the effectiveness of protection against severe illness for those who have received the vaccine. However, in the United States, the majority of severe illnesses and hospitalizations are still unvaccinated people[9], which makes it question how universal and generalizable the situation in Israel is.
On the other hand, according to the CDC statistics of the United States, 70% and 87% of all cases of hospitalization or death caused by breakthrough infections are elderly people over 65 years old [9].
It can be said that the elderly or people with underlying diseases are at greater risk of serious consequences if they develop breakthrough infections. In addition, some studies collected by the CDC show that in places where the elderly gather, such as nursing homes, the effectiveness of the vaccine itself is low and there is a downward trend.
From the perspective of risk and return, there is stronger support for the implementation of booster shot among the elderly.
In contrast to the general population, booster shot not only lacks support for necessity, but it is also difficult to make a judgment that the benefits outweigh the risks. For mRNA vaccines, it is known that there is a risk of myocarditis in young men. Although the incidence is very low, the risk of the second shot is higher than the first shot.
The risk of third dose vaccination is unknown. From the perspective of benefits, for young people, the protection against severe illnesses after vaccination is very good, and there is no downward trend. It is questionable how much further benefits the third dose can bring.
Based on these risk-benefit assessments, FDA experts rejected Pfizer’s application for full-scale booster shot for people over 16 years old, and instead restricted the population to people over 65 years old and other high-risk groups.
On September 22-23, the CDC’s expert meeting responsible for recommending the use of vaccines, on the basis of basically complying with the scope of FDA approval, further restricted to people over 65 years of age or living in long-term care centers, and those aged 18-64 who caused severe illness.
Patients with an increased risk of underlying diseases have rejected those who are at high risk of infection at work within the scope of FDA approval. However, in the official recommendation of the CDC, the director of the CDC, Dr. Walensky, once again included high-risk groups at work.
This difference involves a different starting point in risk assessment. A common reason for including people who are at high risk due to occupation or environment is that medical staff cannot continue to work even if they are mildly ill, which will affect the operation of the entire medical system. .
But in the opinion of CDC’s external experts, the risk-return standard of booster shot should be centered on the vaccinator. For a young front-line worker, the most important personal benefit of playing booster shot-prevention of severe illness, is very low, but the risk, such as rare myocarditis, is there. It cannot be said that he is infected and cannot work.
If it has an impact, I recommend him to do something at a personal level where the risk may outweigh the benefits, not to mention the benefits of booster shot’s prevention of infection among young people are now purely speculative.
The recommendation and non-recommendation here have a certain basis, but more are based on speculation, reflecting the many uncertainties in the specific benefits of booster shot due to limited data.
Effect outlook: Can booster shot change the epidemic?
Although many countries in Europe and the United States have or will implement booster shot for high-age and high-risk groups, the risk-benefit assessment of these groups may also be more probable that the benefits outweigh the risks, but booster shot’s help in overall epidemic control is not necessarily optimistic.
At the FDA’s booster shot expert meeting, CDC’s epidemiologists admitted that the main spread in the United States occurred in unvaccinated people, so booster shot’s containment of the overall epidemic may be limited. This view was also agreed by most of the experts at the conference.
Even among the elderly who may have the greatest direct benefit, the effect of booster shot alone may not be the best. At the CDC meeting on September 22 to 23 to discuss the use of booster shot recommendations, CDC scientists provided a simulation of the effects of booster shot in nursing homes.
If the import risk is high (the infection rate is high in the area) and the vaccination rate of the staff in the facility is low, even if the booster shot effect is very good, there are still many infection cases in the nursing home.
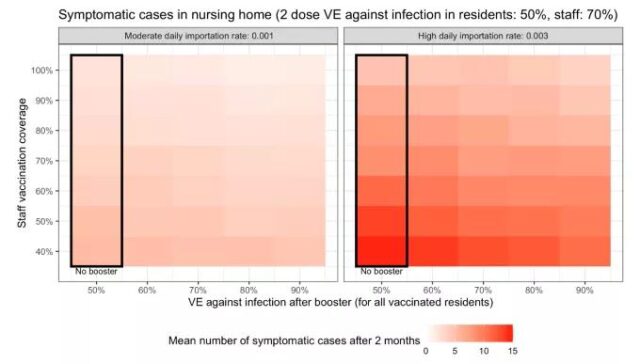 United States CDC on the simulation of the effect of booster shot in nursing homes | Source [10]
United States CDC on the simulation of the effect of booster shot in nursing homes | Source [10]
For booster shot to play a good role, it must be accompanied by controlling the background infection rate and increasing the vaccination rate of the staff. The biggest effect is to increase the vaccination rate of workers. This is also a microcosm of the entire epidemic in Europe and America. Increasing the vaccination rate, allowing more people who have not been vaccinated to get the first shot, the effect will be far greater than the effect of booster shot.
According to CDC’s estimates, to prevent one case of COVID-19 hospitalization within half a year, among people over 65, only 50 initial vaccination is required, but booster shot requires 481 vaccination, which is nearly 10 times the difference [10]. If the age is lowered to 18-29 years old, the gap will be further widened to 22 times.
At this age, even the basic benefits outweigh the risks, and booster shot is not sure. A CDC poll also showed that one-third of the people who have not received the COVID-19 vaccine expressed that the news of booster shot would make them more reluctant to vaccinate [10].
An isolated booster shot policy may seem to have limited harm, but there is uncertainty about what it will be like in all epidemic prevention policies.
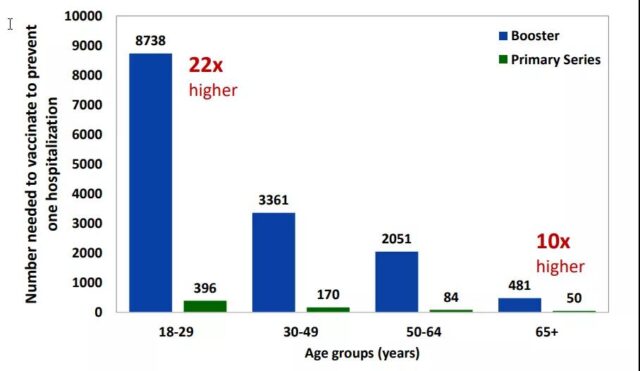 Prevent a case of initial vaccination or booster shot vaccination required for a COVID-19 hospitalization within half a year | Source [10]
Prevent a case of initial vaccination or booster shot vaccination required for a COVID-19 hospitalization within half a year | Source [10]
If you jump out of the small circle of developed countries in Europe and America, it is not difficult to find that booster shot undoubtedly casts a greater shadow on the uneven distribution of global vaccines. Among the vaccinated COVID-19 vaccines, 81% are in high-middle-income countries, and only 0.4% in low-income countries.
COVAX, which is committed to providing vaccines to low-income countries, recently reduced the number of COVID-19 vaccines it can provide this year by a quarter. One [11].
If the epidemic continues to spread in low-income countries with extremely low vaccination rates, it is difficult to guarantee that there will not be a more dangerous mutant than Delta in the future. When proposing the booster shot plan, European and American countries emphasized that it would not affect the global vaccine supply.
However, the global demand for vaccines is far greater than the supply. It is hard to imagine that the large-scale booster shot plan in developed countries will not have a negative impact on global vaccination.
What does booster shot need to do?
Although there are still many doubts about the scientific principles and actual effectiveness of booster shot, in the face of a global epidemic that is difficult to control in the short term, booster shot is still a choice that needs serious consideration.
Even in some people, even if there is uncertainty about booster shot, there will still be real needs now. In the face of this reality, the research and decision-making of booster shot need to be developed in a more scientific and rational direction.
First of all, for the booster shot to be implemented now or in the near future, the actual demand and the potential profit risk must be considered together. Existing booster shot evidence makes it clear that antibodies can be greatly increased in a short period of time after vaccination.
What is the actual effect, if there is an effect, and how long it lasts, is unknown. This makes the benefit that booster shot can bring is very uncertain. In this case, if a person is in a high-risk or high-risk state due to age, underlying disease or occupation, then it may be worth recommending booster shot.
However, if it is extended to the general population, whether it is from the benefit of individual vaccination or the overall epidemic prevention considerations, booster shot may not be worth the gain.
Secondly, the research of booster shot urgently needs to be improved. Among the published booster shot, Pfizer/BioNTech has the most data. But even so, the clinical trial data of this vaccine booster shot only has more than 300 people, and it is impossible to evaluate the rare adverse reactions of known mRNA vaccines such as myocarditis.
In terms of effectiveness, the immunization data is only the antibody titer one month after vaccination. Israel’s real-world data only has a very short tracking time.
More detailed booster shot immune response data, especially changes in memory cells, cellular immunity, and the increased maintenance time of antibodies, can help us clarify whether we should recommend booster shot or change the vaccination program to three shots. .
Different vaccines may also be different. A pre-printed paper of SINOPHARM Vaccine showed that the antibody has improved after the third dose half a year later, and it has been maintained well within three months, and memory cells and cellular immunity have also increased [12].
This may mean that the immune protection brought by the first two injections of the inactivated vaccine can be improved by subsequent vaccination.
However, the number of people in this trial is only 50, which is obviously insufficient to clarify the safety of booster shot and needs follow-up research to supplement it.
And from the perspective of improving immune protection, it is necessary to consider that the audience of booster shot is a group that has already obtained a certain degree of immune protection through the initial vaccination.
The immunogenicity of inactivated vaccines is low. With some more effective vaccines such as Recombinant protein vaccines are on the market, and it should be considered through mixed research to determine a more efficient booster shot.
In the face of a virus that we have known for less than two years and a rapidly changing epidemic, many times our decision-making has to be based on relatively incomplete information, and the same is true for booster shot.
This encourages us to improve relevant research as soon as possible, supplement the completeness of relevant data, and at the same time remind ourselves that we need to be cautious in making decisions based on lack of information. picture
Picture Reference/source:
1.https://www.biorxiv.org/content/10.1101/2021.07.29.454333v1.full
2.https://www.biorxiv.org/content/10.1101/2021.08.23.457229v1.full
3.https://www.science.org/doi/10.1126/science.abj4176
4.https://www.medrxiv.org/content/10.1101/2021.08.09.21261290v4
5.https://www.timesofisrael.com/virus-czar-calls-to-begin-readying-for-eventual-4th-vaccine-dose/
6.https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-september-17-2021-meeting-announcement#event-materials
7.https://www.nejm.org/doi/full/10.1056/NEJMoa2113017
8.https://www.nejm.org/doi/full/10.1056/NEJMoa2114255?query=featured_coronavirus
9.https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html
10.https://www.cdc.gov/vaccines/acip/meetings/slides-2021-09-22-23.html
11.https://www.nytimes.com/2021/09/08/health/covax-global-covid-vaccine-boosters.html
12.https://www.medrxiv.org/content/10.1101/2021.09.12.21263373v1.full-text
Why does U.S. give booster shot to people over 65 but not everyone?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



