Immunotherapy should not for first-line treatment of some cancer patients
- Gut Bacteria Enzymes Offer Hope for ABO Universal Blood Transfusions
- Well-Known Japanese Medicine Exposed for 30 Years of Data Falsification
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
Immunotherapy should not for first-line treatment of some cancer patients
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immunotherapy should not for first-line treatment of some cancer patients.
JCO: For some cancer patients, immunotherapy should not be considered for first-line treatment
Immune checkpoint inhibitors (ICIs), as one of the mainstays of current immunotherapy, seem to have dazzling performance in the treatment of a variety of tumors.
Not long ago, the US FDA has approved nivolizumab and pembrolizumab as the first-line treatment for advanced gastroesophageal adenocarcinoma (GEAC), and pembrolizumab is used for human epidermal growth factor receptor 2 (HER2) positive The treatment of gastric cancer patients has also been quickly approved [1].
However, due to the high degree of heterogeneity of the tumor immune microenvironment, the overall effective rate of immunotherapy for tumors is still not ideal , and the combination of immunotherapy and other treatment options cannot achieve synergistic effects.
In fact, the current researches on ICIs combined with multiple treatments are endless, and people seem to pay more attention to the economic impact of combined immunotherapy.
This also prompts researchers to screen out groups that can really benefit from immunotherapy, so as to truly improve the prognosis of cancer patients.
Previous studies have found that in GEAC patients with high PD-L1 expression, compared with chemotherapy alone, nivolumab combined with chemotherapy can indeed improve the survival of patients [2]. So for GEAC patients with low PD-L1 expression, what is the therapeutic effect of ICIs ?
This question soon ushered in an answer.
Recently, the team of Professor Raghav Sundar from the Cancer Institute of the National University of Singapore published an article in the Journal of Clinical Oncology.
They conducted a retrospective analysis of 3 GEAC clinical cohorts and found that in patients with low PD-L1 expression, chemotherapy was performed +PD-L1 inhibitor compared with chemotherapy alone, the overall survival (OS) and progression-free survival (PFS) of patients were not significantly different (P=0.678) [3].
This result indicates that in GEAC patients with low PD-L1 expression, the combination of ICIs on the basis of chemotherapy did not make the patients have obvious clinical benefits .

Professor Sundar’s team retrieved Phase III clinical studies on immunotherapy in patients with advanced GEAC through databases such as EMBASE, Scopus, PubMed, Web of Science, and ASCO, and performed a step-by-step screening of the results, and only included patients who also included KM Curve and PD-L1 comprehensive positive score (CPS) clinical research cohort.
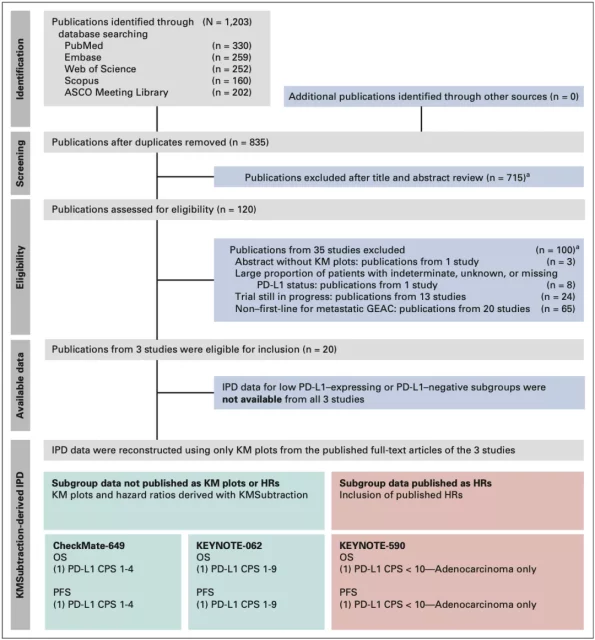
▲ Figure 1: Research PRISMA process chart
In the end, only three clinical cohorts met the requirements: CheckMate-649[2], KEYNOTE-062[4], and KEYNOTE-590[5]. Among them, a small percentage of patients (19/749, 2.54%) in the KEYNOTE-590 cohort lack the PD-L1 CPS score, but it is relatively negligible as a whole.
Next, the researchers selected the subgroups of patients with PD-L1 CPS<1 and CPS<5 in the CheckMate-649 cohort, and reanalyzed the KM curves of their OS and PFS using the KMSubtraction algorithm [6];
in addition, they also Analysis of the esophageal cancer patient group with PD-L1 CPS<10 in the KEYNOTE-590 cohort and the gastric cancer patient group with PD-L1 CPS 1-9 points in the KEYNOTE-062 cohort showed that in each subgroup of patients, the ICIs+chemotherapy group was similar Compared with chemotherapy alone, the OS and PFS were not improved (Figure 2).
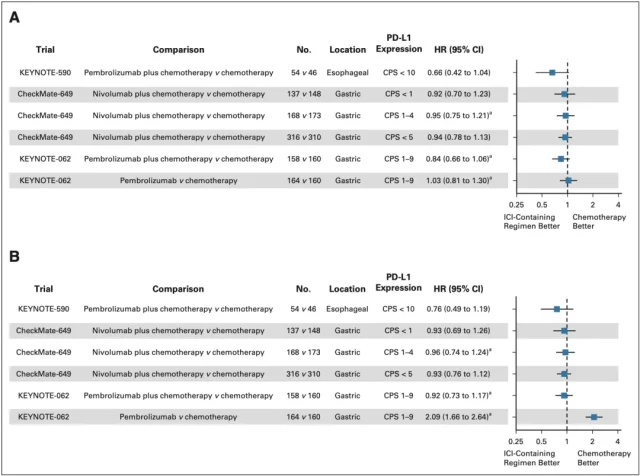
▲ Figure 2: OS (A) and PFS (B) forest plots after reconstruction of KMSubtraction in each clinical cohort subgroup
Specifically, in the PD-L1 CPS 1-4 subgroup of patients in the CheckMate-649 cohort, there was no significant difference in OS between the nivolumab plus chemotherapy group and the chemotherapy alone group (HR=0.950, P=0.678) ; Figure 3A);
Similarly, in the KEYNOTE-062 cohort CPS 1-9 subgroup of patients, compared with chemotherapy alone, pembrolizumab group ( HR=1.027, P=0.827) and pembrolide The improvement of the OS of the patients in the Lizumab combined chemotherapy group (HR=0.836, P=0.141) was not significant (Figure 3B).
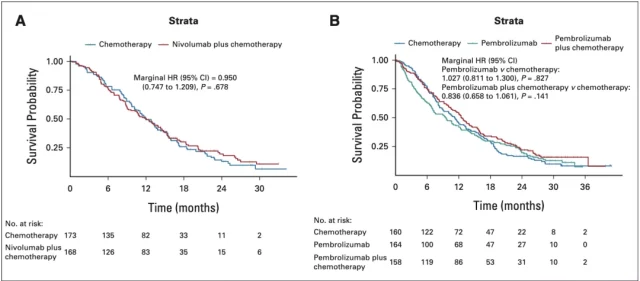
▲ Figure 3: OS after KMSubtraction analysis of the CheckMate-649 and KEYNOTE-062 cohort subgroups
Similarly, in the PD-L1 low expression subgroups of the CheckMate-649 (Figure 4A) and KEYNOTE-062 (Figure 4B) cohorts, the addition of immunotherapy did not prolong the PFS of patients after chemotherapy . In addition, they also found that the PFS of the patients in the pembrolizone single-agent group was significantly shorter than that in the chemotherapy group (HR=2.092, P<0.01).
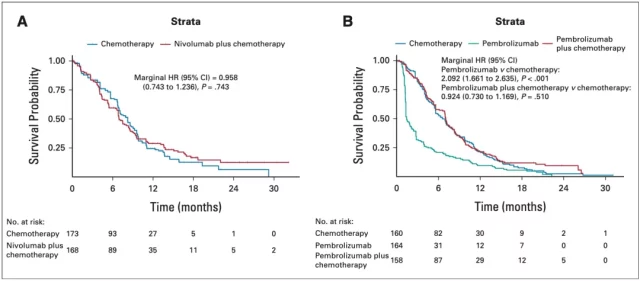
▲ Figure 4: PFS after KMSubtraction analysis of CheckMate-649 and KEYNOTE-062 cohort subgroups
In general, for GEAC patients with low or no PD-L1 expression, the effect of immunotherapy combined with chemotherapy is equivalent to that of chemotherapy alone, and it does not bring more benefits to the patient’s prognosis; while the immunotherapy single-agent treatment group is similar to that of chemotherapy alone.
Compared with chemotherapy alone, the patient’s tumor progression is more significant.
Therefore, researchers believe that the expression of PD-L1 can be used as a biological marker for evaluating the efficacy of ICIs, and the detection of PD-L1 expression will also bring important significance to the formulation of treatment plans.
In GEAC patients with low PD-L1 expression, the combined use of ICIs should be avoided to reduce the side effects and economic burden of immunotherapy; and in patients with high PD-L1 expression, dynamic monitoring of PD-L1 can also be used to assess the effect of ICIs.
The effect of tumor treatment. This study also provides a theoretical basis for the evaluation and selection of first-line treatment options for advanced GEAC patients.
references:
1.Denlinger, C.S., K.A. Matkowskyj, and M.F. Mulcahy, Gastric and Esophageal Cancers: Guidelines Updates. Journal of the National Comprehensive Cancer Network, 2021. 19(5.5): p. 639-643.
2.Janjigian, Y.Y., et al., First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet, 2021. 398(10294): p. 27-40.
3.Zhao, J.J., et al., Low Programmed Death-Ligand 1-Expressing Subgroup Outcomes of First-Line Immune Checkpoint Inhibitors in Gastric or Esophageal Adenocarcinoma. J Clin Oncol, 2021: p. Jco2101862.
4.Shitara, K., et al., Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol, 2020. 6(10): p. 1571-1580.
5.Sun, J.M., et al., Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet, 2021. 398(10302): p. 759-771.
6.Zhao, J.J., et al., KMSubtraction: Reconstruction of unreported subgroup survival data utilizing published Kaplan-Meier survival curves. medRxiv, 2021: p. 2021.09.04.21263111.
Immunotherapy should not for first-line treatment of some cancer patients
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



