China COVID-19 Vaccine: no serious adverse reactions for more than 1 million vaccinations
China COVID-19 Vaccine: no serious adverse reactions for more than 1 million vaccinations
China COVID-19 Vaccine: no serious adverse reactions for more than 1 million vaccinations. Zheng Zhongwei, director of the China National Health Commission’s Medical and Health Technology Development Research Center, said that in order to protect high-risk groups, China approved the emergency use of the COVID-19 vaccine in June, and started the emergency use of the COVID-19 vaccine in July.
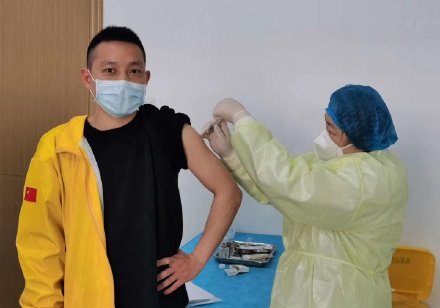
From July to the present, emergency vaccination has been carried out for high-risk exposed populations under the premise of voluntary, informed and consent. At present, more than 1 million doses of emergency vaccination of COVID-19 vaccine have been completed, and strict adverse reaction monitoring and follow-up observation , No serious adverse reactions occurred.
Related previous News:
China SINOPHARM COVID-19 vaccines can reach 1 billion in 2021
China SINOPHARM COVID-19 vaccines can reach 1 billion in 2021. SINOPHARM’s COVID-19 vaccine production capacity can reach 1 billion doses next year.
News from our newspaper (reporter Zhang Hang) Yesterday, Yang Xiaoming, Chairman of SINOPHARM Group China Biotechnology Co., Ltd., introduced that the new coronavirus vaccine developed by Sinopharm Zhongsheng has now carried out Phase III clinical trials in 10 countries and regions, with nearly 60,000 people Entry test. With the completion of the second phase of the workshop this month, the COVID-19 vaccine production capacity will be 1 billion doses in 2021.
At the 2020 academic meeting of the Infectious Disease Specialist Alliance of Beijing Ditan Hospital, Yang Xiaoming said that as of November 26 this year, 48 COVID-19 vaccines worldwide have entered clinical trials, 11 of which have entered phase III clinical trials. In China, 4 vaccines have entered phase III clinical trials, including 3 inactivated vaccines and 1 viral vector vaccine.
“Experiments have proved that the serum of subjects vaccinated with Sinopharm Zhongsheng’s new coronavirus vaccine can produce cross-neutralization protection with many international virus strains, indicating that the vaccine can protect against new coronavirus infections in many places around the world.” Yang Xiaoming said that currently Sinopharm Zhongsheng has carried out Phase III clinical trials in 10 countries and regions, with nearly 60,000 people enrolled, and no enhancement of antibody dependence (ADE phenomenon) has been found.
At present, the combined production capacity of the two workshops of Sinopharm Zhongsheng in Beijing and Wuhan can reach 300 million doses per year. The second phase of the workshop is expected to be completed by the end of the year, with a production capacity of 1 billion doses in 2021
Disclaimer of medicaltrend.org



