Advanced ovarian cancer: First-line treatment of olaparib + immune combination successfully prolongs PFS
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Advanced ovarian cancer: First-line treatment of olaparib + immune combination successfully prolongs PFS
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Advanced ovarian cancer: The first-line treatment of olaparib + immune combination successfully prolongs PFS.
Recently, AstraZeneca announced the positive results of the interim analysis of the DUO-O phase III clinical trial (NCT03737643), suggesting that in patients with newly diagnosed advanced high-grade epithelial ovarian cancer without BRCA mutations, chemotherapy plus bevacizumab (control group) Combination therapy with olaparib, durvalumab, chemotherapy, and bevacizumab resulted in a statistically and clinically meaningful improvement in progression-free survival (PFS) compared to the comparison [1 ] .
This means that for the first-line treatment of patients with advanced ovarian cancer, there is a new effective treatment combination.
 Figure 1 Screenshot from AstraZeneca official website
Figure 1 Screenshot from AstraZeneca official website
Research purposes:
DUO-O is a randomized, double-blind, placebo-controlled, multicenter phase III trial exploring the PD-L1 inhibitor durvalumab in combination with platinum-based chemotherapy and bevacizumab, followed by durvalumab Efficacy and safety of maintenance therapy with riluumab and bevacizumab with or without the PARP inhibitor olaparib in patients with newly diagnosed advanced ovarian cancer without BRCA mutations.
Study design (the study is divided into two groups according to whether patients have BRCA mutation):
① After chemotherapy + durvalumab ± bevacizumab in the BRCA mutation group, durvalumab + olaparib ± bevacizumab was used for maintenance;
② The non-BRCA mutation group was divided into ABC three groups according to 1:1:1:
- Group A was treated with bevacizumab + placebo maintenance therapy after chemotherapy + bevacizumab + placebo;
- After chemotherapy + bevacizumab + durvalumab, groups B and C were given bevacizumab + durvalumab + placebo and bevacizumab + durvalumab respectively. Monoclonal antibody + olaparib for maintenance therapy.
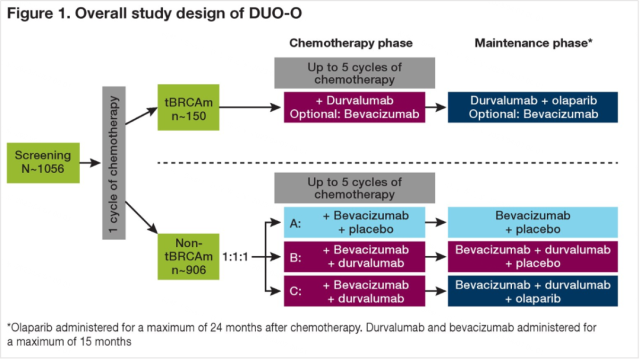 Figure 2 Research design diagram (taken from 2019 ASCO)
Figure 2 Research design diagram (taken from 2019 ASCO)
Research results:
- The interim analysis showed that in the BRCA mutation-free group, compared with the control group (chemotherapy + bevacizumab), the combined therapy of olaparib, durvalumab, chemotherapy and bevacizumab was able to achieve a statistically significant increase in Clinically significantly improved PFS in patients.
- In another treatment cohort, durvalumab plus chemotherapy plus bevacizumab improved PFS in patients compared with control, but this did not reach statistical significance at this interim analysis.
- Overall survival (OS) and other secondary endpoints were immature at the time of the interim analysis and will be further disclosed in subsequent analyses.
Summary
Ovarian cancer is one of the most common gynecological tumors [2] , more than two-thirds of patients are diagnosed at an advanced stage, and usually progress rapidly within two years, and 50%-70% of patients with advanced ovarian cancer will progress within five years. Died within the year [3-6] .
Although significant progress has been made in the treatment of advanced ovarian cancer in recent years, and maintenance therapy with PARP inhibitors has brought significant survival benefits to patients with advanced ovarian cancer, there are still unmet clinical needs.
The combination therapy of olaparib and durvalumab has provided encouraging evidence for patients with advanced ovarian cancer without BRCA mutations.
We look forward to further results in the follow-up, or provide new possibilities for survival benefits for the above-mentioned population.
references:
[1] Lynparza and Imfinzi combination improved progression-free survival in newly diagnosed patients with advanced ovarian cancer without tumor BRCA mutations in DUO-O Phase III trial (astrazeneca.com)
[2] Momenimovahed Z, et al. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int J Womens Health. 2019 Apr 30;11:287-299.
[3] National Cancer Institute. Cancer Stat Facts: Ovarian Cancer. Available at https://seer.cancer.gov/statfacts/html/ovary.html. Accessed March 2023.
[4]Ray-Coquard, et al. Final Overall Survival (OS) Results from the Phase III PAOLA-1/ENGOT-ov25 Trial Evaluating Maintenance Olaparib (ola) plus Bevacizumab (bev) in Patients (pts) with Newly Diagnosed Advanced Ovarian Cancer (AOC). Presented at the European Society of Medical Oncology Congress. Paris, France. 09 September 2022.
[5]Ray-Coquard I, et al. Olaparib Plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019; 381:2416-2428
[6]González-Martín A, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019; 381:2391-2402
Advanced ovarian cancer: First-line treatment of olaparib + immune combination successfully prolongs PFS
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



