Cell: nuclear pore complex | Chromatin | Lymph node | mRNA
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
Cell: nuclear pore complex | Chromatin | Lymph node | mRNA……
Cell: nuclear pore complex | Chromatin | Lymph node | mRNA. Highlights researches that Cell journals have to read in December 2020. What are the highlights of Cell Journal in December that are worth learning?
1. Interpretation of Cell papers! New method reveals in detail the assembly process of nuclear pore complex for the first time
doi:10.1016/j.cell.2020.11.001
In a new study, researchers from the Swiss Federal Institute of Technology Zurich and the University of Bergen in Norway have developed a method that allows them to study the assembly process of large protein complexes in detail for the first time. As their case study, they chose one of the largest cell complexes: the nuclear pore complex in yeast cells.
Related research results were recently published in the Cell Journal, and the title of the paper is “Maturation Kinetics of a Multiprotein Complex Revealed by Metabolic Labeling”. The corresponding authors of the paper are Karsten Weis and Evgeny Onischenko from ETH Zurich.
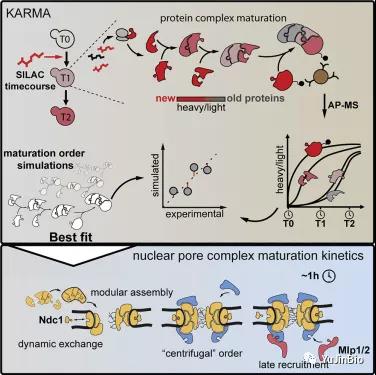
Picture from Cell, 2020, doi:10.1016/j.cell.2020.11.001.
These researchers call their new method KARMA (kinetic analysis of incorporation rates in macromolecular assemblies), which is based on the method of studying metabolic processes. Scientists studying metabolism have long used radiocarbon in their research work, for example, to label glucose molecules, and then cells absorb and metabolize radiocarbon. This radioactive label allows people to track where and when the glucose molecule or its metabolites appear.
2. Detailed Cell Papers! Challenge the routine! Chromatin is neither solid nor liquid, but more like a gel
doi:10.1016/j.cell.2020.11.027
A basic question in genome biology that has plagued scientists since the discovery of DNA: In our cell nuclei, is the complex package of DNA and protein (ie chromatin) solid or liquid? In a new study, from Researchers from the University of Alberta in Canada and Colorado State University in the United States have found the answer to this question.
They found that chromatin is neither solid nor liquid, but more like a gel. Related research results were recently published in the Cell journal, and the title of the paper is “Condensed Chromatin Behaves like a Solid on the Mesoscale In Vitro and in Living Cells”. The corresponding authors of the paper are Michael Hendzel, Professor of Oncology at the University of Alberta, and Jeffrey Hansen of Colorado State University.
Hendzel said that in the past, fields such as biochemistry were conducted under the assumption that chromatin and other components of the cell nucleus were operating in a liquid state. This new understanding of the physical properties of chromatin challenges this perspective and may lead to a more accurate understanding of how the genome is encoded and decoded.
3. Cell: Lymph nodes are innervated by a unique sensory neuron with immunomodulatory potential
doi:10.1016/j.cell.2020.11.028
For a long time, the nervous system and immune system have been considered as independent entities in the body, but a new study has discovered a direct cellular interaction between the two. Researchers from Harvard Medical School, the Broad Institute and the Lagen Institute found that pain neurons surround the lymph nodes in mice and regulate the activity of these lymph nodes, which are a key part of the immune system.
Related research results were recently published in the Cell journal, and the title of the paper is “Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential”.
This new study reveals the cells that mediate the conversation between the nervous system and the immune system. It also paves the way for more research on how the nervous system regulates immune responses.
4. Cell heavy interpretation! The molecular mechanism that obesity damages immune cell function and accelerates tumor growth!
doi:10.1016/j.cell.2020.11.009
Obesity is related to the increased risk of more than a dozen different types of cancer, and it is also directly related to the decline in patient prognosis and survival. Over the years, scientists have identified obesity-related processes that drive tumor growth, such as metabolic changes and chronic inflammation, but they have not elaborated on the specific interaction between obesity and cancer.
Recently, in a research report titled “Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity” published in the international journal Cell, scientists from Harvard Medical School and other institutions have solved this mystery through research. Researchers have found that obesity promotes cancer cells to defeat tumor-killing immune cells in the battle for energy.
Researchers say that a high-fat diet will reduce the number of CD8+ T cells and anti-tumor activity in tumors. This happens because cancer cells reprogram their own metabolism in response to the increase in fat supply, so as to better It swallows energy-rich fat molecules, deprives T cells of fuel, and can accelerate tumor growth.
Researcher Marcia Haigis said that placing the same tumor in an obese and non-obese environment can reveal that cancer cells will rewire their cell metabolism in response to a high-fat diet; related research results indicate that it may be effective in a certain environment The therapy may not be so effective in another environment. Given the current prevalence of obesity in the population, it may require further research and understanding by scientists.
Blocking fat-related metabolic reprogramming may significantly reduce the tumor volume of mice on a high-fat diet. Since CD8+ T cells are the main weapon for immunotherapy to activate the host’s immune system against cancer, the researchers proposed improvements in this study. New strategies for this type of therapy. Cancer immunotherapy can have a huge impact on the lives of cancer patients, but not every patient can benefit.
Now researchers know that as obesity changes, there is a metabolic tug of war between T cells and tumor cells. This study may provide a roadmap to explore this interaction, which may help us start thinking about cancer in a new way. The mechanism of action of immunotherapy and combination therapy.
5. Detailed Cell Papers! I am in charge of my destiny, and so do the RNA molecules in the cell
doi:10.1016/j.cell.2020.11.030
Scientists have a good understanding of how transcription begins: a protein called RNA polymerase is recruited to a specific area of a DNA molecule and begins to move along the DNA strand, synthesizing mRNA molecules while walking. However, part of this process is not clear: how does the cell know when to stop transcription?
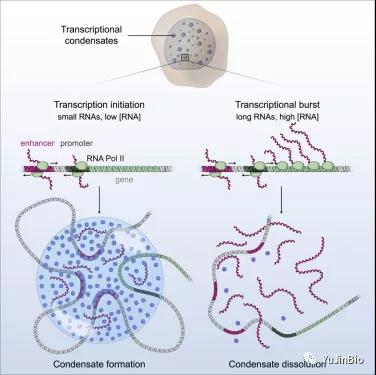
Picture from Cell, 2020, doi:10.1016/j.cell.2020.11.030.
Now, in a new study, Richard Young and his team, a member of the Whitehead Institute of Biomedical Research and a professor of biology at MIT, and Arup K. Chakraborty, professor of chemical engineering at MIT, and his team have discovered that RNA molecules pass Feedback loops regulate their own formation. If there are too few RNA molecules, the cell will initiate transcription to produce more RNA molecules.
Then, at a certain threshold, too many RNA molecules will cause transcription to stop. The relevant research results were published online on December 16, 2020 in the Cell Journal, with the title of the paper “RNA-Mediated Feedback Control of Transcriptional Condensates”.
6. Cell: Subvert traditional cognition! The mRNA in the cell stress granule can indeed express protein
doi:10.1016/j.cell.2020.11.010
Just like people, cells are also stressed. Sudden drop in oxygen, overheating, or toxins will trigger a series of molecular changes that cause cells to stop growing, produce stress protection factors, and form stress granules — no membrane formed by squeezing protein and RNA molecules together Organelles.
Although the function of stress particles is still largely unknown, it is thought that they only contain RNA that is not translated into protein. Now, a new study has overturned this long-standing view, showing that messenger RNA (mRNA) in stress particles can indeed make proteins.
The relevant research results were published online on December 11, 2020 in the Cell Journal, with the title of the paper “Single-Molecule Imaging Reveals Translation of mRNAs Localized to Stress Granules”.
In order to find out what happens to the mRNA in these organelles caused by stress, the corresponding author of the paper, Dr. Jeffrey Chao, the first author of the paper, and the postdoctoral researcher Daniel Mateju, and colleagues began to observe the relationship between single RNA molecules and living cells undergoing stress Interaction between stress particles. To this end, they labeled the stress particles and individual mRNA molecules with fluorescent tags. Thanks to an innovative antibody labeling tool called SunTag, they can also visualize the protein production with single-molecule precision.
Using this method, these researchers found that even if mRNA is stably located in the stress granule, it can still be translated into protein. Although the translation of most mRNAs is inhibited during stress, specific genes (such as ATF4) that are necessary to trigger the stress response increase their translation under these conditions.
By using ATF4-SunTag as a model transcript, they found that its translation in the stress granule is not a rare event, and the complete translation cycle (initiation, extension, termination) can occur in the stress granule.
In addition, they also found evidence that mRNA whose translation is inhibited during stress can also be translated in stress granules.
7. Two Cell papers revealed that potent human neutralizing antibodies are expected to resist the attacks of Eastern Equine Encephalitis Virus, Hendra Virus and Nipah Virus
doi:10.1016/j.cell.2020.11.011; doi:10.1016/j.cell.2020.11.023
Although COVID-19 has grabbed the headlines, scientists are making steady progress in the fight against other dangerous viruses. These include Eastern Equine Encephalitis Virus (EEEV), Hendra virus (HeV) and Nipah virus (Nipah virus, NiV). Eastern equine encephalitis virus is one of the most toxic viruses in North America. Hendra virus and Nipah virus are endemic in Southeast Asia.
Like COVID-19, there is currently no way to stop these scourges. However, in two papers published this week in the journal Cell, Dr. James Crowe Jr. of Vanderbilt University Medical Center and his colleagues have made new progress in developing human monoclonal antibodies that potentially treat and prevent these infections. progress.
In the first paper, these researchers reported that two potent neutralizing antibodies, EEEV-33 (IgG) and EEEV-143, were isolated from survivors of natural eastern equine encephalitis virus infection. (IgA) — Protect mice from the deadly attack of this virus aerosol. The relevant research results were published online on December 9, 2020 in the Cell Journal, with the title of the paper “Human Antibodies Protect against Aerosolized Eastern Equine Encephalitis Virus Infection”.
In the second paper, they discovered the first naturally occurring human monoclonal antibodies that target the Hendra virus receptor binding protein. All of these antibodies can neutralize Hendra virus, some of them can also neutralize Nipah virus, including the two most potent cross-reactive neutralizing antibodies: HENV-26 and HENV-32. The relevant research results were published in the Cell Journal on December 10, 2020. The title of the paper is “Potent Henipavirus Neutralization by Antibodies Recognizing Diverse Sites on Hendra and Nipah Virus Receptor Binding Protein”.
8. Interpretation of Cell papers! The new discovery subverts the traditional understanding of killer T cells and helps develop CAR-T cells that better target solid tumors
doi:10.1016/j.cell.2020.11.019
A class of immune cells called “killer T cells” is also called cytotoxic or cytolytic CD8 T cells. In a new study, researchers from the Perelman School of Medicine at the University of Pennsylvania found that killer T cells usually stay in the blood and do not enter organs and other tissues. The relevant research results were published online on December 10, 2020 in the Cell journal, with the title of the paper “The Identity of Human Tissue-Emigrant CD8+ T Cells”.
This discovery may help solve many problems in immunology, including medically significant mysteries, such as why the recently developed cancer therapy using genetically modified killer T cells does not work well against solid tumors, and why The AIDS-causing virus, which is thought to be very vulnerable to killer T cells, seems to be able to evade these immune cells indefinitely by hiding out of the blood.
The co-corresponding author of the paper, Dr. Michael Betts, professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania, said, “This discovery tells us that killer T cells usually do not migrate out of the blood. Now that we know this, we can start Design treatments that make better use of these powerful cells.”
9. Major progress! Two Cell papers reveal host factors necessary for the proliferation and spread of coronavirus and flavivirus
doi:10.1016/j.cell.2020.12.005; doi:10.1016/j.cell.2020.12.006
In two new studies, researchers from research institutions such as New York University and Rockefeller University found that a protein necessary for the SARS-CoV-2 coronavirus to proliferate and spread to other cells is a potential weakness that future therapies may target . This protein, called transmembrane protein 41 B (TMEM41B), is believed to help shape the fatty outer membrane that protects the viral genetic material before the virus replicates in an infected cell and infects another cell.
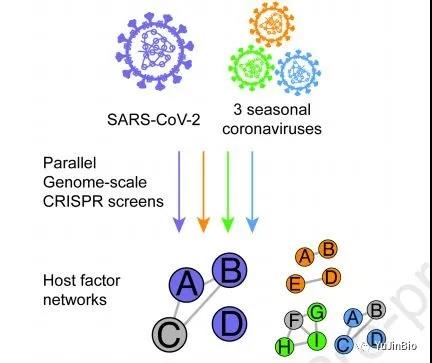
Picture from Cell, 2020, doi:10.1016/j.cell.2020.12.006.
These authors found that TMEM41B is essential for the replication of SARS-CoV-2. In the first study, through a series of experiments, they combined the proliferation of the SARS-CoV-2 virus that causes COVID-19 in infected cells with more than two dozen deadly flaviviruses (including yellow fever virus, The same processes in West Nile virus and Zika virus) were compared. The relevant research results were published online in Cell on December 8, 2020, with the title of the paper “TMEM41B is a pan-flavivirus host factor”.
In the second study, they also compared the way SARS-CoV-2 multiplies in infected cells with three other seasonal coronaviruses known to cause the common cold (HCoV-OC43, HCoV-NL63 and HCoV-229E) Make a comparison. The relevant research results were published online on December 9, 2020 in the Cell Journal, with the title of the paper “Genome-scale identification of SARS-CoV-2 and pan-coronavirus host factor networks”.
10. Detailed explanation of heavy Cell papers! Quantitative detection of new coronavirus RNA within 30 minutes using CRISPR-Cas13a and smartphone cameras
doi:10.1016/j.cell.2020.12.001
In a new study, the researchers outlined a CRISPR-based COVID-19 test technology that uses a smartphone camera to provide accurate results within 30 minutes. The relevant research results were published online in Cell on December 4, 2020, with the title of the paper “Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy”.
In this new test method, the Cas13a protein is combined with a reporter molecule that fluoresces when cut, and then mixed with a patient sample from a nasal swab. The mixed sample is placed in a device connected to a smartphone. If the sample contains SARS-CoV-2 RNA, Cas13 will be activated and cleave the reporter molecule, resulting in a fluorescent signal. Then, the smartphone camera, which basically turns into a microscope, can detect the fluorescence and report that the tested swab is positive for the virus.
Dr. Melanie Ott, co-corresponding author of the paper and director of the Glaston Institute of Virology, said, “The real uniqueness of this test method is that it uses a one-step reaction to directly test viral RNA instead of the two-step process in traditional PCR testing. A simpler chemical reaction, coupled with a smartphone camera, shortens the test time and does not require complex laboratory equipment. It also allows this test method to produce quantitative measurement results instead of simple positive or negative results.”
These researchers also said that their test method can be applied to various mobile phones, thus making it easy to use. Fletcher explained, “We chose to use mobile phones as the basis of our detection equipment because of their intuitive user interface and highly sensitive cameras that allow us to detect fluorescence. Mobile phones are also mass-produced and cost-effective. , Which shows that specialized laboratory equipment is not necessary for this test method.”
Cell: nuclear pore complex | Chromatin | mRNA
(source:internet, Cell: nuclear pore complex | Chromatin | mRNA, reference only)
Disclaimer of medicaltrend.org



