The location and role of T cell response in SARS-CoV-2 infected mice
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
The location and role of T cell response in SARS-CoV-2 infected mice
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
The location and role of T cell response in SARS-CoV-2 infected mice. Virus-specific T cells (including SARS-CoV and MERS-CoV) play an important role in resisting various viral infections.
Although SARS-CoV-2 specific T cells have been identified in COVID-19 patients, their role in protecting SARS-CoV-2 infected mice has not yet been determined. Here, we used an adenovirus expressing the human receptor (Ad5-hACE2) to transduce mice sensitive to SARS-CoV-2 infection.
In BALB/c and C57BL/6 mice, we identified CD4+ and CD8+T SARS-CoV-2 specific T cell epitope recognized by cells. Virus-specific T cells are multifunctional and can lyse target cells in the body.
In addition, after SARS-CoV-2 infection, the type I interferon pathway proved to be the key to optimal antiviral T cell responses. Vaccination of T cell vaccine alone can partially protect SARS-CoV-2 infected mice from severe diseases.
In addition, the results of the study showed that there is a cross-reactive T cell response between SARS-CoV and SARS-CoV-2 in mice, but there is no cross-reaction between MERS-CoV and SARS-CoV-2. Understanding the role of T cell response will guide the research on the immune pathogenesis of COVID-19 and the design and verification of vaccines.
Introduction:
A new β-coronavirus, Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2), was reported in Wuhan in December 2019. The pathogen of the 2019 coronavirus disease was released by the World Health Organization.
An international public health emergency was declared in January 2020. As of December 5, 2020, more than 65 million people have been infected worldwide and more than 1 million deaths.
Virus clearance during primary respiratory virus infection depends on the production of effective virus-specific CD4+ and CD8+ T cell responses. Although respiratory virus infections sometimes cause transient antibody responses, virus-specific T cells tend to be more persistent. For example, among SARS survivors, SARS coronavirus-specific T cells can be detected 17 years after infection.
MERS-CoV-specific T cell responses have been detected in most patients, including asymptomatic patients whose antibody response cannot be detected. Recently, SARS-CoV-2 specific T cells have been reported in COVID-19 patients during the acute and convalescent phases, and these T cells are closely related to the severity of the disease.
In addition, cross-reactive T cells were found in unexposed individuals with SARS-CoV-2, which suggests that pre-existing cross-reactive T cells against seasonal respiratory coronaviruses or unidentified pathogens may be immune to COVID-19. Play a role in the mechanism.
Mice are ideal animals for studying pathogenesis and evaluating new therapies and vaccines. Our previous study identified SARS-CoV and MERS-CoV-specific CD4+ and CD8+ T cells in mice through the expression of IFN-γ after epitope peptide stimulation. CD4+ and CD8+ T cell deficient SCID and RAG-/- mice have delayed virus clearance.
The decrease of SARS-CoV-specific CD8+ T cell response in the lungs of old mice is related to the increase of mortality. In infected C57BL/6 and BALB/c mice, MERS-CoV-specific CD8+ T cells are also required to clear the virus. These results together support the key role of T cells in clearing the virus in CoV-infected mice.
Recently, we used replication-deficient adenovirus (Ad5-hACE2) to exogenously deliver human ACE2 (hACE2) to establish a COVID-19 mouse model. Ad5 transduction can support CoV replication in mouse lungs 17-22 days after transduction.
Ad5-hACE2 sensitized mice developed pneumonia, manifested by weight loss, severe lung disease, high-titer virus replication in the lungs, and a strong antiviral adaptive immune response. Ad5-hACE2 transgenic mice can study immune pathogenesis and quickly evaluate antiviral drugs and vaccine candidates.
So far, no CD4+ or CD8+ T cell epitopes have been found in SARS-CoV-2 infected mice, and the functional identification of virus-specific T cells is incomplete.
Here, we used Ad5-ACE2 transgenic mice to identify SARS-CoV-2 specific T cell epitopes in BALB/c and C57BL/6 mice. We found that SARS-CoV-2 specific CD4+T and CD8+T cells have versatility and cytosolubility. Inoculation of T cells without neutralizing antibodies can protect mice from SARS-CoV-2 infection.
In addition, there is a cross-reactive T cell reaction between SARS-CoV and SARS-CoV-2 in mice, but there is no cross-reaction between MERS-CoV and SARS-CoV. This study provides a basis for immune pathogenesis research, vaccine design and in vivo verification.
Results:
Identification of CD4+ and CD8+ T cell epitopes in WT-BALB/c and C57BL/6 mice infected with SARS-CoV-2
In order to locate MHC class I-restricted CD8+ and MHC class II-restricted CD4+ T cell epitopes, a group of four SARS-CoV-2 structural proteins (spike [S] glycoprotein, nucleocapsid [ N] protein, transmembrane [M] and envelope [E] protein) 20 peptides.
These peptides overlap by 10 amino acids. Initially, the expression SARS-CoV-2-S, SARS-CoV-2N, SARS- Venezuelan equine encephalitis replicon particles (VRPs) of CoV-2M, SARS-CoV-2E, ORF3a, ORF6, ORF7a and ORF9c.
Mice were inoculated with VRP in, 7 days after inoculation, cells were separated from the lungs, and 5μM of each 20 peptides or 10μM of each protein peptide mixture was used in brefeldinA (brefeldin; protein Stimulate for 5-6 hours in the presence of transport inhibitors.
Antigen-Use intracellular cytokine staining (ICS) to identify specific T cells. For BALB/c (H-2d restricted) mice, compared with the no peptide control group, six peptides (N-7, N-25, N-36, S-7, S-45 and F8-5;
Figure S1 A) and four peptides (N-9, S-27, S-54 and S-106; Figure S1 B) can stimulate CD4+ and CD8+ T cells to produce IFN-γ, respectively. For C57BL/6 (H-2b-restricted) mice, seven peptides (N-1, S-3, S-7, ORF3a-16, ORF3a-27, ORF3a-28 and ORF7a-7; Figure S2 A ) And 24 peptides (M-18, N-11, N-22, N-23, N-28, S-8, S-10, S-13, S-14, S-22, S-23, S-24, S-26, S-27, S-48, S-51, S-52, S-54, S-55, S-57, S-82, ORF3a-11, ORF7a-9 and ORF7a- 10); Figure S2 B) can respectively stimulate CD4+ and CD8+ T cells in VRP-immunized mice. It is worth noting that the VRP vaccine can induce high levels of exogenous gene expression and effectively induce humoral and T cell responses in mice, as we have previously described (Sunet al., 2020; Zhao et al., 2016).
Using our localization method is unlikely to miss dominant T cell epitopes; however, we cannot rule out this possibility. Use T cell epitope consensus server (Rankpep, Immune Epitope Database and Analysis Resource, and SYFPEITHI) to further analyze these peptides.
A series of truncated peptides were synthesized, and the lung cells of BALB/c and C57BL/6 mice were used to identify precise epitopes. As shown in Figure S1 (C and D) and Figure S2 (C and D), the truncated peptide that induced the strongest T cell response in the same group of 20 peptide truncated peptides was selected as the candidate T cell epitope peptide .
To confirm these epitopes, BALB/c and C57BL/6 mice were transduced with Ad5-ACE2, and 5 days later, 1×105 PFU SARS-CoV-2 was used for i.n. infection. Bronchoalveolar lavage fluid (balf) was taken 8 days after infection, and the cells were stimulated with the indicated ccandidate epitope peptides.
ICS method was used to determine the effect of IFN-γ production on T cell response. A total of 6 I-Ad-restricted CD4+ T cell epitopes (Figure 1A) and 3 H-2K/D/Ld-restricted CD8+ T cell epitopes were confirmed (Figure 1B; BALB/c mice) , 5 I-Ab-restricted CD4+ T cell epitopes (Figure 1C) and 10 H-2K/Db-restricted CD8+ T cell epitopes (Figure 1A; C57BL/6 mice).
It is worth noting that some candidate epitopes found in mice vaccinated with VRP did not appear in SARS-CoV-2 infected mice, which may reflect differences in viral protein expression levels in infected mice or epitopes in vivo Direct competition between.
Table 1 summarizes the epitopes and their MHC restrictions. Select the dominant CD4+ T cell epitope N351-365 (N351) in BALB/c mice, ORF3a 266-280 (ORF3a 266) in C57BL/6 mice, and dominant CD8 in BALB/c mice +T cell epitope S535-543 (S535) and S538-546 (S538) in C57BL/6 mice were further studied.
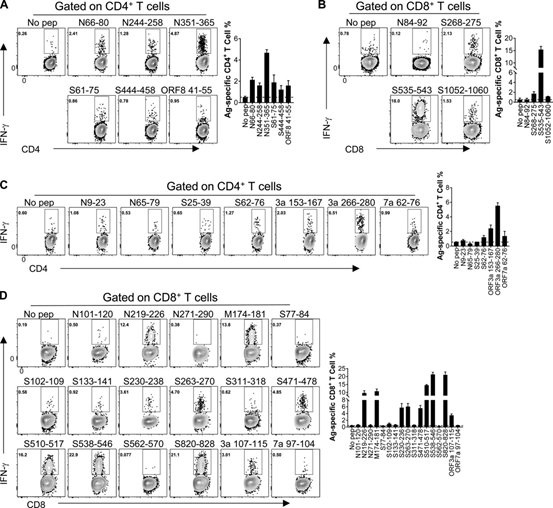
Figure 1 The identification of CD4+ and CD8+ T cell epitopes in WT-BALB/c and C57BL/6 mice infected with SARS-CoV-2
(A and B) CD4+ T cell epitope (A) and CD8+ T cell epitope (B) in infected BALB/c mice. (C and D) CD4+ T cell epitope (C) and CD8+ T cell epitope (D) in infected C57BL/6 mice. The flow chart and histogram are shown (n=3; data verified in two independent experiments). All results are expressed as mean±SEM. Ag, antigen; pep, polypeptide.
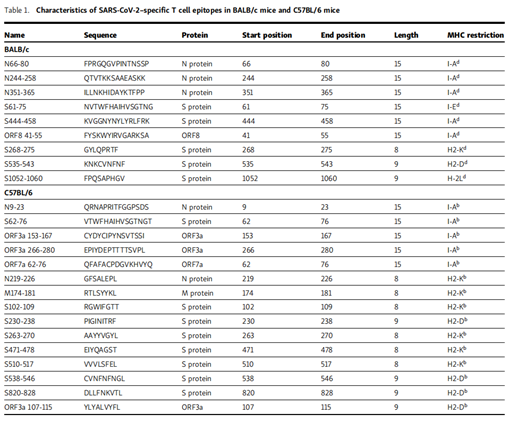
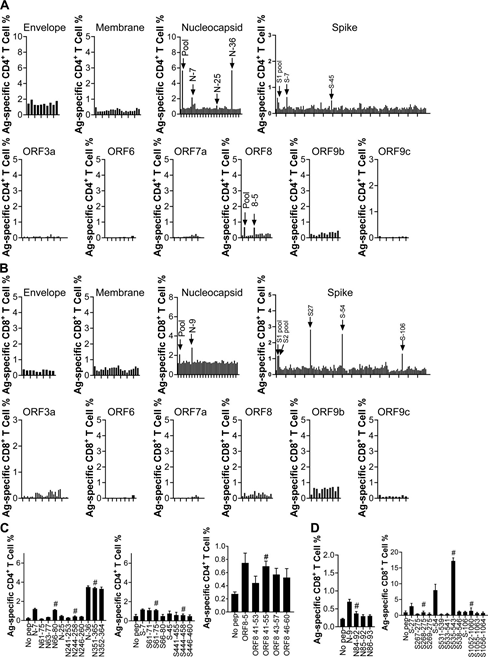
Figure S1: Localization of SARS-CoV-2t cell epitopes in BALB/c mice.
(A) A 5 µM 20-mer (20 amino acid) peptide was used to stimulate lymphocytes from the inoculated lung. Intracellular IFN-γ staining detects antigen-specific CD4+ T cell response.
(B) A 5 µM 20-mer (20 amino acid) peptide was used to stimulate lymphocytes from the inoculated lung. Detection of antigen-specific CD8+ T cell response.
(C) Lymphocytes from the inoculated lung were stimulated with 5 µM 20-mer (20 amino acids) and the corresponding truncated 13-15-mer peptide in the presence of brefeldin A for 5-6 hours. The antigen-specific CD4+ T cell response was measured.
(D) Lymphocytes from the inoculated lung were stimulated with 5 µM 20-mer (20 amino acids) and the corresponding truncated 8-9-mer peptide in the presence of brefeldin A for 5-6 hours.
The antigen-specific CD8+ T cell response was measured. Candidate truncated epitopes are marked with # (n=3; data represents at least two independent experiments). All results are expressed as mean±SEM. Ag, antigen; pep, polypeptide.
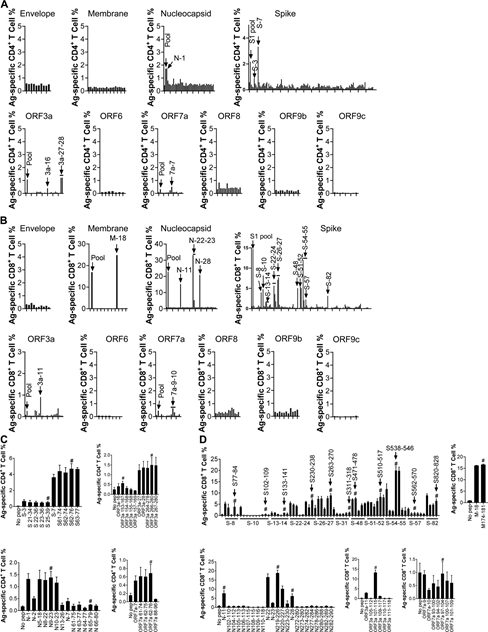 Figure S2: Localization of the epitope on C57BL/6 mouse SARS-CoV-2t cells.
Figure S2: Localization of the epitope on C57BL/6 mouse SARS-CoV-2t cells.
(A) A 5 µM 20-mer (20 amino acid) peptide was used to stimulate lymphocytes from the inoculated lung. Intracellular IFN-γ staining detects antigen-specific CD4+ T cell response.
(B) A 5 µM 20-mer (20 amino acid) peptide was used to stimulate lymphocytes from the inoculated lung. Detection of antigen-specific CD8+ T cell response.
(C) Stimulate lymphocytes from the inoculated lung with 5 µM 20-mer (20 amino acids) and the corresponding truncated 13-15-mer peptide. Detection of antigen-specific CD4+ T cell response.
(D) The lymphocytes from the inoculated lung were stimulated with 5µM 20-mer (20 amino acids) and the corresponding truncated 8-9-mer peptide.
Detection of antigen-specific CD8+ T cell response. Candidate truncated epitopes are marked with # (n=3; data represents at least two independent experiments). All results are expressed as mean±SEM. Ag, antigen; pep, polypeptide.
The kinetics of virus-specific T cell response in SARS-CoV-2 infected BALB/c and C57BL/6 mice
In order to understand the dynamic changes of SARS-CoV-2 specific T cell response after SARS-CoV-2 infection, the infected wild-type BALB/c and C57BL/6 mice were sacrificed at 5, 8 and 10 d.p.i. Cells were collected from BALF, lung tissue, lung drainage LNs (DLNs) and spleen, and stimulated with the indicated dominant CD4+ or CD8+ T cell epitope peptide.
The frequency of IFN-γ+CD4+T cells recognizing N351 peptide peaked at 8-10 dpi, and it was found in the airway (Figure 2A), lung tissue (Figure 2B), DLN (Figure S3A) and spleen of BALB/c mice (Figure S3B) The maximum number of cells was observed at 8 dpi.
SARS-CoV-2–S535–specific CD8+ T cell response peaked on the 8th day after infection of BALB/c mice, and contracted rapidly from the 8th day (Figure 2, c and d; Figure S3c and Figure S4 d). The kinetics of SARS-CoV-2-specific CD4+ and CD8+ T cell responses were directed against ORF3a 266 (Figure 2, E and F; Figure S3, E and F) and S538 (Figure 2, G and I; Figure S3, respectively) G and I) Peptides, similar to the trends observed in the airways, lung tissue, DLN of BALB/c mice, and spleens of transduced/infected C57BL/6 mice.
The frequency of SARS-CoV-2 specific T cells in the respiratory tract is much higher than that in the lung, DLN and spleen. Virus-specific T cells in the airway that first encounter viral antigens are essential for mediating protection after SARS-CoV and MERS-CoV attacks (Zhao et al., 2016).
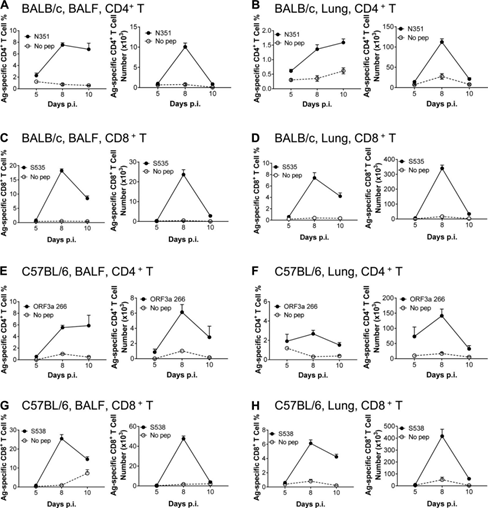
Figure 2: SARS-CoV-2 infection of BALB/c and C57BL/6 mice BALF and virus-specific T cell response kinetics. (A–D) Lymphocytes were collected from the airways and lungs of transduced/infected WT BALB/c mice at designated time points after infection, and used 5µM N351 (A and B) and 1µM S535 in the presence of brefeldinA (C and D) Stimulate for 6 hours. BALF (A and c) and the frequency (left) and the number of cells (right) D) of antigen-specific T cells in the lungs (B and D) are shown (n=3 or 4 mice; data represent three independent experiments). (E-H) Collect the airway and lung lymphocytes of transduced/infected C57BL/6 mice at the specified time points, and use 5μM ORF3a 266 (E and F) and 1μM S538 (G and H) in the presence of brefeldin A ) Stimulate for 6 hours. Shows the frequency (left) and cell number (right) of antigen-specific T cells (n=3 or 4 mice; data represents three independent experiments). All results are expressed as mean±SEM. Ag, antigen; pep, polypeptide; p.i., after infection.
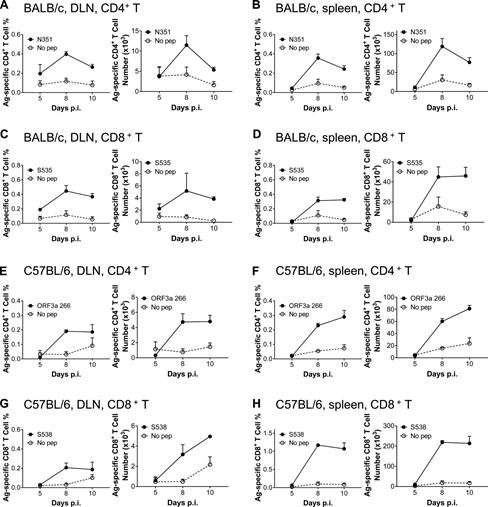
Figure S3: Response kinetics of virus-specific T cells in dln and spleen of BALB/c and C57BL/6 mice infected with SARS-CoV-2. (A–D) Lymphocytes were obtained from the DLN and spleen of transduced/infected WT BALB/c mice at designated time points after infection, and used 5µM N351 (A and B) and 1µM S535 in the presence of brefeldin A (C and D) Stimulate for 6 hours. The frequency (left) and number of cells (right) D) of antigen-specific T cells in DLN (A and c) and spleen (B and D) are shown (n=3 mice; data represents one experiment). (E–H) Lymphocytes were collected from the DLN and spleen of transduced/infected C57BL/6 mice at the designated time points, and 5µM ORF3a 266 (E and F) and 1µM S538 (G and H) Stimulate for 6 hours. Shows the frequency (left) and cell number (right) of antigen-specific T cells (n=3; data represents an experiment). All results are expressed as mean±SEM. Ag, antigen; pep, polypeptide; p.i., after infection.
SARS-CoV-2 specific CD4+ T cells and CD8+ T cells have versatility
Next, our goal is to describe the phenotype and function of SARS-CoV-2 specific T cell responses, especially infecting cells in the airways of mice. The IFN-γ expression method was used to detect the airway N351-CD4+T cells and S535-CD8+T cells of transfected/infected BALB/c mice, and the virus-specific cell phenotype was detected by flow cytometry.
The activation markers (CD44, CD43, CD11a, CD49d, CD27 and CD69) were upregulated as expected after encountering antigen on N351-CD4+ T cells (Figure 3A) and S535-CD8+ T cells (Figure 3E).
Adhesion molecules (CD11a and CD49d) and chemokine receptors (CXCR3, CXCR6, and CCR5) are also up-regulated, which may help T cell migration and localization to the airway (Figure 3, A and E).
The expression levels of L-selectin (CD62L) and chemokine receptor (CCR7) that guide T cells into LNs are reduced (Figure 3, A and E).
CD103 and Ly6C are expressed on S535-CD8+ T cells, but not on N351-CD4+ T cells, which is consistent with previous studies (Figure 3, A and E).
Functional markers (CD107a/b) related to T cell cytotoxicity were expressed on N351-CD4+ T cells and S535-CD8+ T cells, which is different from the observations of N351-specific CD4+ T cells and S535-specific Sexual CD8+ T cells can lyse peptide-pulsed target cells in vivo (Figure 3, D and H).
In addition to CD107a/b, CD40L is believed to be associated with CD4+ T cell-mediated killing, while CD4+ T cells are not expressed on virus-specific CD8+ T cells (Figure 3, A and E).
Our findings indicate that CD4+ T cells are cytotoxic, which is also consistent with previous reports. Inhibitory molecules (CTLA-4 and PD-1) were up-regulated, while low levels of KLRG1 and high expression of CD127 were observed on N351-CD4+ T cells, but higher on S535-CD8+ T cells (Figure 3, A And E).
The expression of phenotypic markers on S538-CD8+ T cells of C57BL/6 mice is similar to that of S535-CD8+ T cells of BALB/c mice (Figure S4A).
Airway N351-specific CD4+ T cells and S535-specific CD8+ T cells have superior effector functions, which can produce more than three cytokines (IFN-γ, TNF, IL-10 and IL-2) at the same time (Figure 3, B and F).
S538-specific CD8+ T cells in C57BL/6 mice mainly synergistically produce IFN-γ and TNF (Figure S4B). Virus-specific T cells that produce IL-10 were also found in SARS-CoV-2 infected mice, which is consistent with the study of influenza A virus.
In addition, virus-specific CD4+ T cells and CD8+ T cells in the respiratory tract showed greater functional affinity than those in the lung, indicating that the specific T cells in the respiratory tract were more sensitive to virus antigen mimicking (Figure 3, C And G; and Figure S4 C).
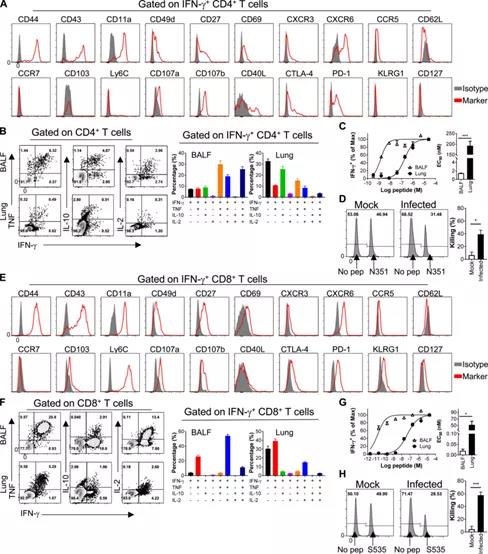
Figure 3: SARS-CoV-2 specific CD4+ T cells and CD8+ T cells have versatility. (A and E) Phenotypic analysis of SARS-CoV-2-N351-specific CD4+ T cells (A) and SARS-CoV-2-S535-specific CD8+ T cells (E) derived from BALF. (B and F) show the cytokine expression of airway-derived N351-specific CD4+ T cells (B) and S535-specific CD8+ T cells (F) (n=3 mice; data represents two independent experiments ).
(C and G) shows the functional affinity curves of airway and lung-derived N351-specific CD4+ T cells (C) and S535-specific CD8+ T cells (G) (left) and the amount of peptide required for the half-maximal response (EC50) (right; n=3 mice; data represents two independent experiments; Student’s T test; P value of C is 0.0004; P value of G is 0.0051).
(D and H) show SARS-CoV- 2 Representative flow histograms (left) and killing rate (right) of cytotoxicity of N351-specific CD4+ T cells (D) and S535-specific CD8+ T cells (H) in infected mice and mock-infected mice (n = 5 mice/group; data represents two independent experiments; Student’s T test; D has a P value of 0.0062; H has a P value of 0.0002). *, P<0.05; ***, P<0.0005. All results are expressed as mean±SEM. Max, maximum value; pep, polypeptide.
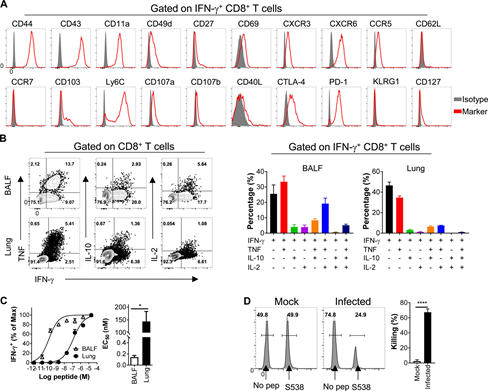
Figure S4: SARS-CoV-2 specific CD8+ T cells infected with C57BL/6 mice are multifunctional.
(A) BALF cells are stained with antibodies against the indicated markers. The histogram shown here is gated on SARS-CoV-2-S538-specific CD8+ T cells.
(B) shows the cytokine expression of SARS-CoV-2-S538-specific CD8+ T cells derived from the respiratory tract (n=3 or 4 mice; data represents an experiment).
(C) shows the functional affinity curve of airway and lung-derived SARS-CoV-2-S538-specific CD8+ T cells (left) and the amount of peptide required for half-maximal response (EC50) (right; n=3) ; Data represents two independent experiments; StudentT test; C’s P value is 0.011).
(D) shows the representative flow histogram of SARS-CoV-2-S538-specific CD8+T cytotoxicity in SARS-CoV-2-infected mice and mock-infected mice (left) and killing rate (right) (N=5; data represents an experiment; Student’s T test; P value of C<0.0001). ****, P<0.0001. All results are expressed as mean±SEM. Maximum, maximum; pep, polypeptide.
Type I IFN (IFN-I) signal transduction is the key to a strong T cell response against SARS-CoV-2 infection
IFN-I is an important part of innate immunity, triggering an “antiviral state” in infected tissues. IFN-I also affects the antiviral adaptive immune response, including virus-specific T cell response.
COIVD-19 patients with weakened immune function often have more serious diseases and poor prognosis. In order to clarify the role of IFN-I in T cell response after SARS-CoV-2 infection, we studied N351-CD4+ T cells and S535-CD8+ in IFNAR (type I IFN receptor) KO-BALB/c mice T cell dynamics and cytokine production. Like wild-type BALB/c mice, virus-specific CD4+ and CD8+ T cell responses peaked on day 8 after infection (Figure 4, A and B).
However, the frequency and cell number of N351-specific CD4+ T cells and S535-specific CD8+ T cells in IFNAR KO BALB/c mice were significantly lower than those in WT mice (Figure 4, c and D).
In addition, although some IFNAR KO T cells are determined to be multifunctional by expressing two cytokines (IFN-γ combined with TNF or IL-10 or IL-2), the frequency of bifunctional and multifunctional cells is lower than that of WT mice (Figure 4, E and F).
In addition, the functional affinity of virus-specific CD4+ T cells and CD8+ T cells in IFNAR KO mice is lower than that of WT mice (Figure 4, G and H), indicating that the loss of IFN-I signal reduces virus-specific T cells.
The sensitivity of cells to antigens may be due to changes in the inflammatory environment of different strains of mice or the increased expression of viral antigens in IFNAR-KO mice. IFNAR-KO mice have delayed weight gain and decreased virus clearance kinetics in the late stage of infection (days 4-6; Fig. 4I).
This may be related to impaired T cell response, because T cell response starts at this time after infection. The lungs appear. These results indicate that IFN-I signal is necessary for SARS-CoV-2 specific T cell development and function optimization.
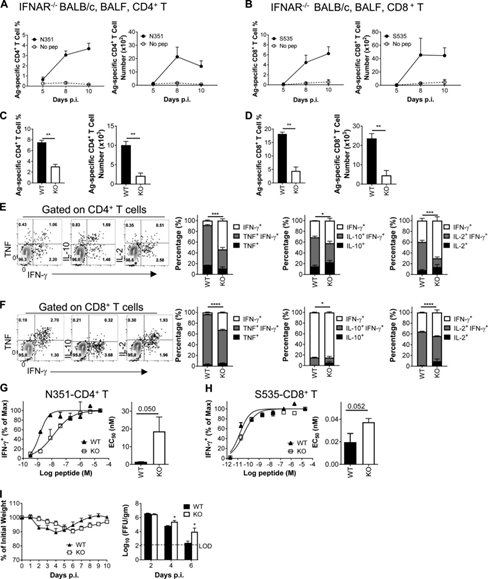
Figure 4: IFN-I signal is essential for generating a strong T cell response against SARS-CoV-2 infection. (A and B) Show the frequency (left) and cell number (right) of airway-derived N351-specific CD4+ T cells (A) and S535-specific CD8+ T cells (B) at the specified time point (n=3) Or 4 mice/time point; data represents three independent experiments).
(C and D) Comparison of airway-derived N351-specific CD4+ T cell responses (C) and S535-specific CD8+ T cell responses (D) in WT and KO BALB/C mice (n=3 or 4 mice ; Data represents two independent experiments; StudentT test; P values of C are 0.0006 and 0.0013; P values of D are 0.0005 and 0.0029). (E and F) shows representative flow diagrams (left) of N351-specific CD4+ T cells (E) and S535-specific CD8+ T cells (F).
Two-cytokine expression ability (the right three groups) is statistically different between WT and KO mice (n=3 or 4mice; data represents two independent experiments; Student’s t-test; P values of E are 0.0003, 0.0057 and respectively) 0.0004; P values of F are <0.0001, 0.0445 and <0.0001). (G and H) shows the functional affinity curves of N351-specific CD4+ T cells (G) and S535-specific CD8+ T cells (H) in KO and WT mice (left) and the amount of peptide required for the half-maximal response (EC50) (right; n=3 mice; data represents two independent experiments; Student T test; P value of G is 0.050; P value of H is 0.052).
(1) Measure weight loss and lung virus titer at designated time points (n=3–5 mice/group/time point virus titer; data represents two independent experiments; Student t test; P value 0.0291 and 0.0394). *, P<0.05; **, P<0.005; ***, P<0.0005; ***, P<0.0001. All results are expressed as mean±SEM. Ag, antigen; FFU, lesion forming unit; Max, maximum value; pep, peptide; p.i., after infection.
Epitope-specific CD4+ and CD8+ T cells partially protect SARS-CoV-2 infected mice from severe disease
Next, we assessed whether SARS-CoV-2 specific T cells alone can protect mice from infection. As mentioned earlier, a set of VRP vectors expressing only a single immunodominant T cell epitope were produced, including VRP-N351 (I-Ad, BALB/c), VRP-S535 (H-2Dd, BALB/c) and VRP-S358 (H-2Db, C57BL/6).
Take VRP-GFP as control. Mice were immunized twice every 4 weeks and infected on the 28th day after the boost (Figure 5a). The balf was harvested at the specified time point (Figure 5a).
Intracellular IFN-γ staining detects epitope-specific T cells. Vaccination with VRP-N351, S353 and S358 without VRP-GFP can induce epitope-specific T cell responses in the airway, which are enhanced by enhancement and are strongly recalled after infection.
After immunization, it was observed that the SARS-CoV-2 titer in the mouse lung was moderately reduced, and the pathological changes in the lung tissue were alleviated (Figure 5, B-D).
Neutralizing antibodies from different VPR vaccination groups were below the detection limit at challenge, and there was no difference in the half-maximum neutralizing titers in the focus reduction neutralization test (FRNT50) at 14 dpi (Figure S5), indicating that it was virus-specific Cells, rather than neutralizing antibodies, have a protective effect.
These results indicate that the vaccine-induced virus-specific T cells mediate faster virus clearance in SARS-CoV-2 infected mice and reduce the degree of lung lesions.
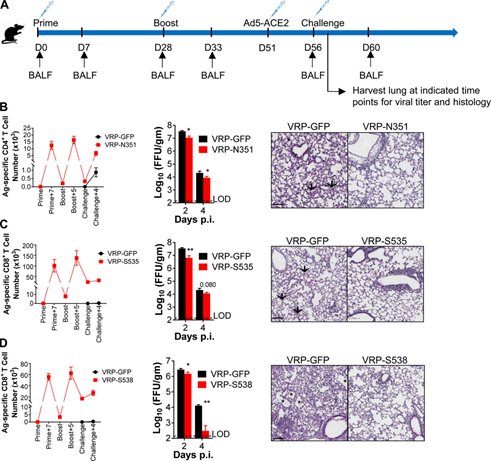
Figure 5. Epitope-specific CD4+ and CD8+ T cells partially protect SARS-CoV-2 infected mice from severe diseases. (A) VRP vaccination strategy and SARS-CoV-2 challenge.
(B and C) The effects of N351-specific CD4+ T cells (B) and S535-specific CD8+ T cells (C) on BALB/C mice. Shows the number of airway-derived antigen-specific T cells (n=3 or 4 mice/group/time point; left).
At the specified time point (n=4 mice/group/time point; middle; data represents at least two independent experiments; Student t-test; P values of B are 0.0051 and 0.0403; P values of C are 0.0026 and 0.0804) Measure lung virus titer.
Hematoxylin/eosin was used to stain paraffin-embedded lung sections of infected mice (n=3 mice per group at each time point; right; data represents at least two independent experiments).
Scale bar, 100 mm. (D) The effect of S538-specific CD8+ T cells on C57BL/6 mice. Display the number of cells at the specified time point (n=3 mice/group/time point; left).
The lung virus titer was measured at the designated time points (n=4 mice/group/time point; middle; data represents at least two independent experiments; Student t-test; D values are 0.0372 and 0.0043).
Six days after infection, paraffin-embedded lung sections of infected mice were stained with hematoxylin/eosin (n=3 mice/group/time point; right; data represents at least two independent experiments).
Scale bar, 100 mm. *, P<0.05; **, P<0.005. All results are expressed as mean±SEM. Arrow, bleeding; asterisk, edema; Ag, antigen; D, day; FFU, lesion forming unit; p.i., after infection.
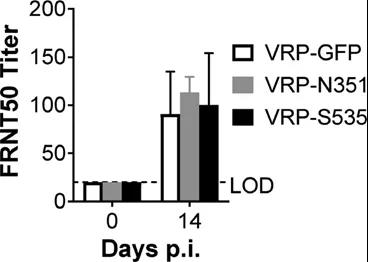
Figure S5: BALB/c mice have no neutralizing antibodies when challenged. Inoculated and infected BALB/c bmice serum neutralizing antibody titer at challenge and 14 d.p.i. (n=6; data represents an experiment). All results are expressed as mean±SEM. LOD, detection limit; p.i., after infection.
There is a cross-reaction between SARS-CoV-2 and SARS-CoV infection in mice
SARS-CoV-2, SARS-CoV and MERS-CoV are three highly pathogenic human respiratory coronaviruses belonging to the genus β-coronavirus.
The homology of SARS-CoV-2 genome to SARS-CoV and MERS-CoV is 79.6% and ∼40%, respectively, indicating that cross-reactive T cell epitopes may exist. However, the same and cross-reactive T cell epitopes have not been described.
Previously, we have identified all T cell epitopes in all four structural proteins in SARS-CoV infected mice, as well as several T cell epitopes in MERS-CoV infected BALB/c mice. This study compares the number of infections.
The T cell epitope of SARS-CoV-2 in mice and the T cell epitope of SARS-CoV and MERS-CoV in BALB/c and C57BL/6 mice. Figure 6a summarizes the same epitopes and possible cross-reactive T cell epitopes.
In BALB/c mice, the core sequence of the dominant CD4+ T cell epitope of SARS-CoV (N353-370) and SARS-CoV-2 (N351-365) is the same, and is the same as that of MERS-CoV (N350 -362) The core sequence of the dominant CD4+ T cell epitope is different. Between SARS-CoV (S521-529) and SARS-CoV-2 (S535-543; Figure 6a), there is an amino acid difference in the dominant CD8+ T cell epitope. In C57BL/6 mice, the three CD8+ T cell epitopes of SARS-CoV-2 are the same as SARS-CoV.
No conserved T cell epitopes between SARS-CoV-2 and MERS-CoV were found in C57BL/6 mice (Figure 6a).
Next, in SARS-CoV-2 infected mice, it was tested whether these potential cross-reactive T cell epitopes actually induced cross-reactive T cell responses. The CD4+ T cells in the BALF of SARS-CoV-2 infected mice respond to SARS-CoV-2 (N351-365) and SARS-CoV (N353-370) peptide stimulation, but to MERS-CoV (N350-362) No peptide response (Figure 6B).
SARS-CoV (S521-529) peptide successfully stimulated the CD8+ T cells of SARS-CoV-2 infected BALB/c mice, with a cross-reaction rate of >70% (Figure 6c).
As expected, when stimulated with S535-543 peptide, cross-reacting SARS-CoV-2 (S535-543) CD8+ T cells showed higher functional affinity than SARS-CoV (S521-529) peptide (Figure 6 D).
These data clearly demonstrate the existence of a cross-reactive T cell response between SARS-CoV and SARS-CoV-2 infections in BALB/c mice. Whether SARS patients and COVID-19 patients have similar cross-reactive T cell responses remains to be further studied.
Image 6. In SARS-CoV-2 infected mice, there is a cross-reactive T cell reaction between SARS-CoV-2 and SARS-CoV. (A) The characteristics of conserved T cell epitopes in SARS-CoV-2, SARS-CoV and MERS-CoV. (B and C) BALB/C mice are infected with SARS-CoV-2.
Lymphocytes from the airway were prepared at 8d.p.i. and stimulated with the transformed epitope. IFN-γ expression was tested for CD4+ (B) and CD8+ T cell response (C).
The flow graph (left) and cross-reaction rate (right) are shown (n=3 or 4 mice per group; data represents two independent experiments).
(D) shows the functional affinity curve of S535-specific CD8+ T cells and S521-cross-reactive CD8+ T cells (left) and the amount of peptide required for half-maximal response (EC50) (right; n=3 mice ; Data represents two independent experiments; Student’s t-test; P value of D is 0.0182). *, P<0.05. All results are expressed as mean±SEM. Max, maximum value; pep, polypeptide.
Discuss:
The COVID-19 pandemic is a serious public health threat and has led to a massive increase in patients hospitalized with pneumonia and multiple organ dysfunction. The immune pathogenesis is still unclear, which hinders the development of new preventive and therapeutic measures, including vaccine design and validation.
Many respiratory viruses require T cells to clear, including SARS-CoV and MERS-CoV. CoV-specific T cells play an indirect or direct role in virus clearance and immunity. In SARS-CoV-infected mice, airway memory CD4+ T cells promote airway dendritic cell migration by up-regulating CCR7 expression, and activate stati pathway by producing IFN-γ, leading to a more powerful cytotoxic CD8+ T cell response, and Increase the expression of chemokine CXCL9-11 at the site of infection to track CD8+ T cells that are more virus-specific. However, in SARS-CoV-2 infected mice, T cell epitopes are still unknown.
It is the key to understanding immune pathogenesis and validating vaccines and therapies. We have identified T cell epitopes in SARS-CoV-2 infected mice and used a peptide library containing all four structural proteins and six accessory proteins, including six of BALB/c and C57BL/6 mice I-Ad restricted, 3 H2-K/Dd restricted, 5 I-Ab restricted and 10 H2-K/Db restricted T cell epitopes.
In BALB/c mice (N351) and C57BL/6 mice (orf3a266), dominant CD4+ T cell epitopes are located in the N protein. In BALB/c mice (S535) and C57BL/6 mice (S538), dominant CD8+ T cell epitopes are located in S protein.
The selection of epitopes recognized by T cells is restricted by MHC, so the epitopes recognized by T cells in BALB/c and C57BL/6 mice are different. The CD8+ T cell response of C57BL/6 mice is stronger than that of BALB/c mice, which may help C57BL/6 mice to clear the virus faster.
Respiratory virus-specific T cells provide the first line of defense against challenges and enhance the immune response early after infection. Compared with lung parenchymal cells, airway-derived SARS-CoV-2 specific CD4+ T cells and CD8+ T cells are superior effector cells, which is consistent with previous studies.
In IFNAR-KO mice, the frequency and number of total and multifunctional virus-specific T cells and the sensitivity to antigen stimulation are reduced, leading to delayed virus clearance, which indicates that IFN-I signal is optimized for SARS-CoV-2 -The functions necessary for the production and expression of specific T cells are consistent.
With this, COVID-19 patients with low immune function have a higher viral load, hospitalization rate, intensive care unit admission rate, and severe respiratory diseases.
In order to explore whether virus-specific memory T cells can mediate long-term protection alone, we constructed a VRP vector expressing a single SARS-CoV-2 specific CD4+ or CD8+ T cell epitope. 4wk after mice were inoculated with VRP, the virus titer decreased moderately and the lung lesions were alleviated, which can partially protect the mice from SARS-CoV-2 infection.
This experiment provides direct evidence for the protective effect of memory T cells.
Finally, this study identified several cross-reactive T cell epitopes between SARS-CoV and SARS-CoV-2 in BALB/c and C57BL/6 mice, but MERS-CoV was not identified. Neither SARS-CoV-2 nor SARS-CoV has a licensed vaccine.
A durable vaccine can induce extensive protection against multiple coronaviruses, which will be useful and may provide long-term protection for other SARS-like coronaviruses that may emerge in the future.
In conclusion, we proved that SARS-CoV-2 induced a strong T cell response in mice. In this study, T cell epitopes were identified in both BALB/c and C57BL/6 mice. SARS-CoV-2 specific CD4+T and CD8+T cells are multifunctional, able to lyse target cells in vivo, and protect mice from SARS-CoV-2 infection in the absence of neutralizing antibodies. In addition, the IFN-I pathway proved to be the key to the best antiviral T cell response after SARS-CoV-2 infection.
In addition, there is a cross-reactive T cell reaction between SARS-CoV and SARS-CoV-2 in mice, but there is no cross-reactive T cell reaction between MERS-CoV. The identification of T cell epitopes and the study of T cell response characteristics not only contribute to the study of immune pathogenesis of COVID-19 disease in mice, but also contribute to vaccine design and in vivo verification.
The location and role of T cell response in SARS-CoV-2 infected mice
The location and role of T cell response in SARS-CoV-2 infected mice
(source:internet, reference only)
Disclaimer of medicaltrend.org



