SINOPHARM COVID-19 Vaccines Approved and Free in China
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
SINOPHARM COVID-19 Vaccines Approved and Free in China
SINOPHARM COVID-19 Vaccines Approved and Free in China. The COVID-19 vaccine is free for all people! Sinopharm China Biotech Covid-19 Inactivated Vaccine Approved in China!
On December 31, the joint prevention and control mechanism of the State Council of China issued, and the Sinopharm China Bio-COVID-19 Inactivated Vaccine has been approved by the State Food and Drug Administration for conditional listing. Yesterday, the interim analysis data of its phase III clinical trial was released, and the data showed that its safety and effectiveness are good. In the future, the durability and protective effect of vaccine immunity need to be continuously observed.
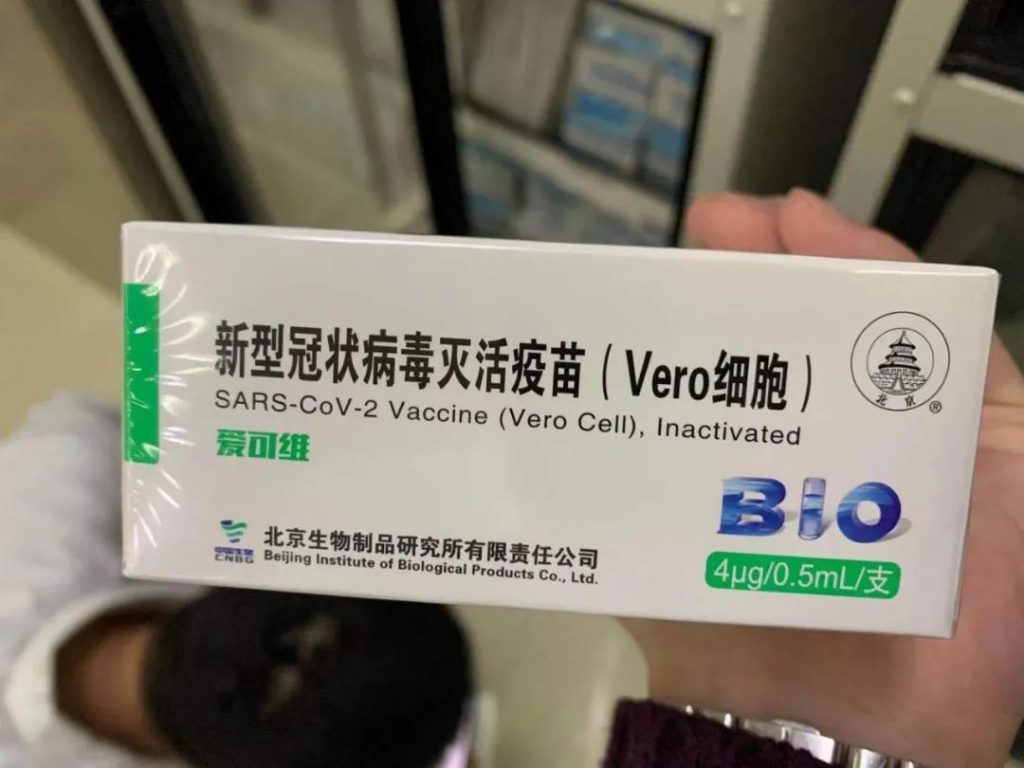
On December 31, on the release of the Joint Prevention and Control Mechanism of the State Council of China, Zeng Yixin, deputy director of the National Health Commission and head of the vaccine research and development team of the Joint Prevention and Joint Control Mechanism of the State Council, said that the COVID-19 virus vaccine must be provided free of charge to all people.
The results of the interim analysis showed that Sinopharm’s COVID-19 vaccine was safe after inoculation. After two injections of the immunization program, all vaccinators in the vaccine group produced high-titer antibodies, and the neutralizing antibody positive conversion rate was 99.52%. The vaccine was targeted by the new coronavirus infection. The protective effect of COVID-19 is 79.34%, and the data results meet the relevant technical standards of the World Health Organization and the relevant standards in the “Guidelines for Clinical Evaluation of New Coronavirus Preventive Vaccines (Trial)” issued by the State Food and Drug Administration.
According to reports, China National Biotech has prepared for large-scale production. Two high-grade biosafety production workshops for the COVID-19 vaccine, Beijing Institute of Biological Products and Wuhan Institute of Biological Products, have been completed. The production capacity is expected to reach more than 1 billion doses next year. Ensure a safe and adequate supply of vaccines.
At the State Council Joint Prevention and Control Press Conference held today, Wu Yonglin, President of Sinopharm China Biotechnology, gave answers on the situation of Sinopharm China’s bio-COVID-19 inactivated vaccine.
Effectiveness and durability of inactivated COVID-19 vaccine
Sinopharm Sinopharm has conducted antibody durability observations in both domestic phase I/II clinical studies and overseas phase III clinical studies. According to phase I/II clinical studies of the COVID-19 inactivated vaccine, observation data for more than 6 months shows that antibodies are still Maintain at a high level. The United Arab Emirates and Bahrain have reviewed and approved the official registration and listing of Sinopharm’s China Bio-COVID-19 Vaccine in accordance with the relevant technical standards of the World Health Organization. The clinical trial data results show that the protective data results have reached the predetermined target and met the registration and listing requirements. Currently, clinical studies in China, the UAE, and Bahrain are still ongoing, and the persistence of antibodies will continue to be monitored.
According to some countries’s conditional listing work plan and the relevant technical standards of the World Health Organization, the results of protective data observed so far have reached the predetermined target and meet the conditional listing requirements. The COVID-19 vaccine is an innovative vaccine, and the durability and protective effect of immunity need to be observed for a longer period of time. As the Phase III clinical trial continues, the effectiveness of the vaccine will continue to be observed and long-term protection rate data will be obtained.
COVID-19 inactivated vaccine production capacity
The high-level biosafety production workshops of the COVID-19 inactivated vaccines established by Sinopharm Group in Beijing and Wuhan respectively have been put into large-scale production after inspection and certification by the relevant state departments. The Beijing production base has a designed annual production capacity of 120 million doses. It is under expansion and the production capacity is expected to reach 1 billion doses next year. In accordance with the relevant deployment requirements, China Biotech will also make overall plans to further expand production capacity to better meet demand.
How to objectively evaluate the COVID-19 inactivated vaccine?
The large-scale phase III clinical studies of Sinopharm Sinopharm’s COVID-19 inactivated vaccine in the UAE, Bahrain and other countries have nearly 60,000 people inoculated. The sample size of the vaccinated population covers 125 nationalities, and the phased protection efficiency evaluation has been completed. The results obtained are better than the predetermined goals of clinical research, and the safety and effectiveness indicators exceed the requirements of the World Health Organization’s listing standards and the conditional listing work plan approved by some countries, which can form effective protection in a large range of populations.
On December 9th and 13th, the UAE and Bahrain reviewed and approved the official registration and marketing of Sinopharm’s China Bio-COVID-19 Vaccine in accordance with the relevant technical standards of the World Health Organization.
The conditional listing of the COVID-19 vaccine will inject confidence in the world’s eventual victory over the epidemic, provide strong support for the realization of the COVID-19 vaccine’s accessibility and affordability as a global public product, and make China’s contribution.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



