China COVID-19 vaccines Recognized by Mexico
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
China COVID-19 vaccines Recognized by Mexico
China COVID-19 vaccines Recognized by Mexico. The effectiveness and convenience of China’s COVID-19 vaccine are recognized by the President of Mexico.
On January 3, 2021, the official WeChat account of the “People’s Daily” posted “Thank you to China”. Mexican Foreign Minister Ebrard expressed on social media that he thanked the People’s Republic of China for its strong support to Mexico in fighting the COVID-19 pneumonia epidemic; Mexican President Luo Pace recently praised the effectiveness and portability of the Cansino (COVID-19) vaccine, which will bring hope to the elderly in Mexico and residents living in remote areas.
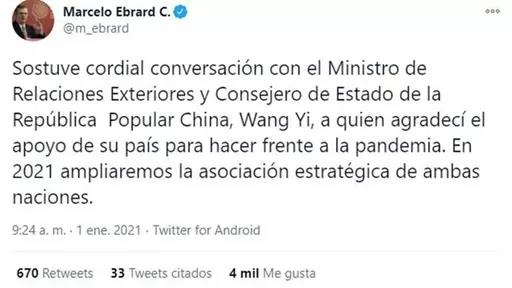
(Mexico Foreign Minister posted on social media)
According to previous reports, in October 2020, a new coronavirus vaccine from China has been approved by the Mexican drug regulatory agency; in November 2020, the vaccine will be launched in Mexico for phase III clinical trials, and all subjects in the first group will be enrolled And get vaccinated. So, who is the research and development team behind this vaccine? Which technical route is used? How is effectiveness and convenience reflected? Let’s take a look together.
It is reported that this COVID-19 vaccine was jointly developed by the team of Academician Chen Wei of the Academy of Military Sciences and CanSino Bio. The full name is the recombinant adenovirus vector COVID-19 vaccine (Ad5-nCoV). It was officially launched on January 22, 2020 and on March 16. The start of clinical trials is the world’s first COVID-19 vaccine to enter clinical trials and the only third-generation vaccine in China to enter phase III clinical trials.

(Photo of Academician Chen Wei’s Laboratory)
The recombinant adenovirus vector COVID-19 vaccine (Ad5-nCoV) uses adenovirus vector technology. With this technology, the team of Academician Chen Wei of the Cansino Biological and Military Science Academy successfully developed a recombinant Ebola virus disease vaccine (Ad5-EBOV) in 2017. ), therefore, adenovirus vector technology is a proven safe and effective technology platform.
As a genetically engineered vaccine, in addition to the above-mentioned safety, the advantage of this vaccine is that it can induce both humoral and cellular immunity. If the immune response process is likened to a war between the immune system and foreign enemies, then the immune response induced by this vaccine is equivalent to a joint combat by sea, land and air to completely eliminate the enemy, and the immunogenicity is naturally better. Based on the immunological principles of COVID-19 vaccine candidates based on different technical routes, not all vaccines can stimulate cellular immunity.
How does this vaccine work?
In short, the adenovirus vector new coronavirus vaccine (Ad5-nCoV) constructs the gene of the new coronavirus S protein into the adenovirus genome. The coat is still the normal coat protein of adenovirus, but the gene inside contains the gene encoding the new coronavirus S protein. Therefore, when adenovirus infects the host cell, it releases the gene encoding the new coronavirus S protein into the host cell, synthesizes the S protein in the cytoplasm, and the S protein stimulates a series of immune responses.
The results of the Phase II clinical trial of the vaccine showed that after 28 days of a single vaccination of the adenovirus vector COVID-19 vaccine, 97% of the subjects developed specific antibodies and 88% of the subjects developed specific cellular immunity. This shows that the vaccine can provide double protection to subjects, and one shot of vaccination can play a protective role.

(Screenshot of Phase II clinical trial paper)
This vaccine only needs one shot, which not only provides convenience for the vaccinators, but also greatly reduces the burden and cost of medical institutions. In addition, the vaccine can be stored at 2~8℃, and ordinary cold chain transportation is sufficient. These are the reasons for the convenience of vaccines mentioned by the President of Mexico.
(source:internet, reference only)
Disclaimer of medicaltrend.org



