SNF472 reduces cardiovascular calcification in hemodialysis patients
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
SNF472 reduces cardiovascular calcification in hemodialysis patients
SNF472 reduces cardiovascular calcification in hemodialysis patients. Can SNF472 reduce the progression of cardiovascular calcification in hemodialysis patients? This is a phase IIb clinical trial with reasonable design and comprehensive follow-up.

Background
- High cardiovascular morbidity and mortality in hemodialysis patients
- Atherosclerosis and calcification of the middle layer of arteries lead to extensive calcification of the cardiovascular system, which can partially explain the risk
- SNF472 is a myo-inositol hexaphosphate that inhibits the formation of hydroxyapatite crystals, which is the last step of vascular calcification
Test Participants:
- Hemodialysis patients (18-80 years old), of whom 18-54 years old must have a history of diabetes
- Dialysis time> 6 months
- Coronary artery calcium Agatston score is between 100 and 3500
Intervention/control
Placebo group vs. SNF472 300mg group vs. SNF472 600mg group
Test flow chart
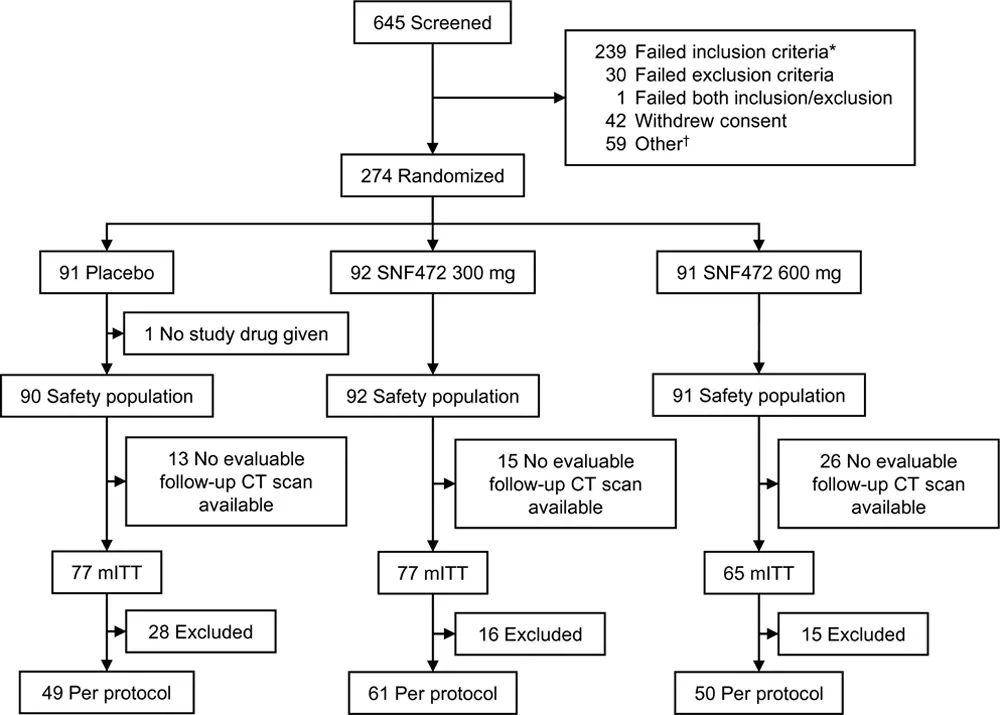
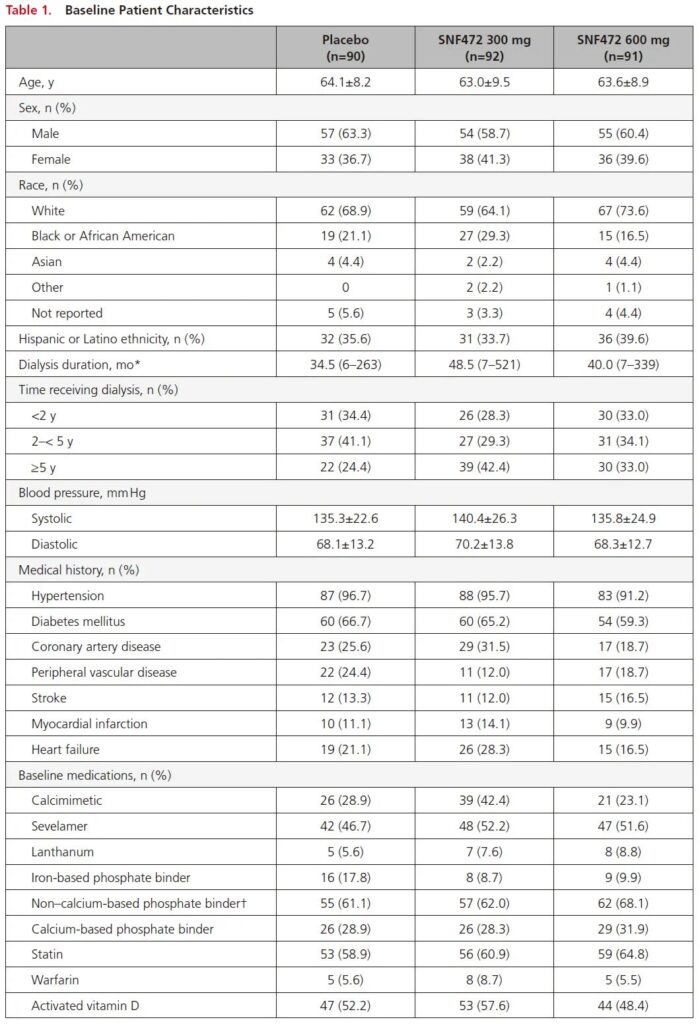
Baseline data
Patients’ baseline clinical data, the three groups are basically the same
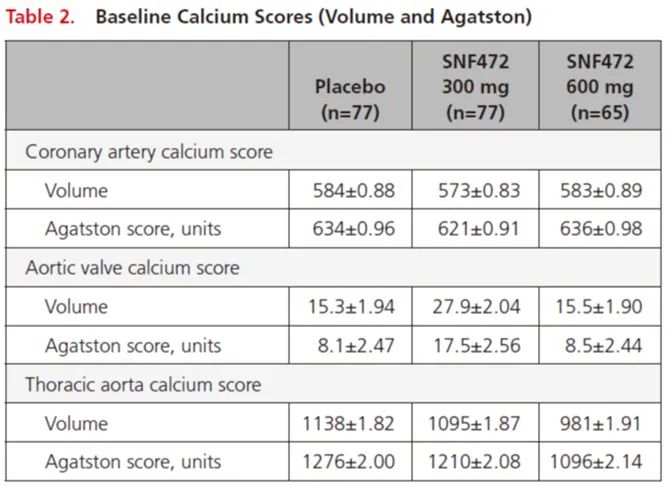
There was no difference in calcification between the three groups at baseline
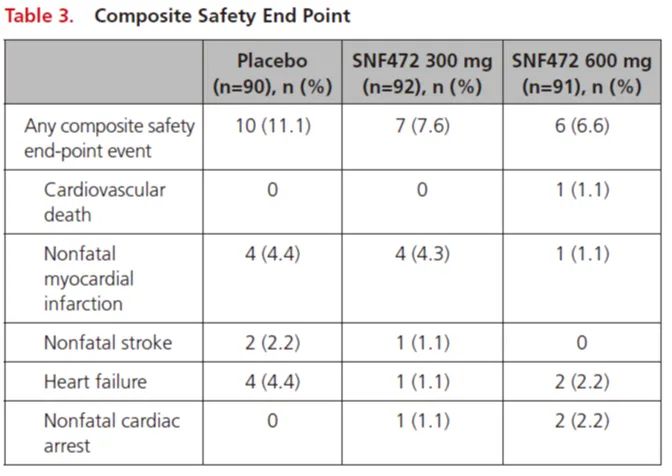
Assessment
The primary end point event is the log value of the coronary artery calcium volumes core from 0 to 52 weeks
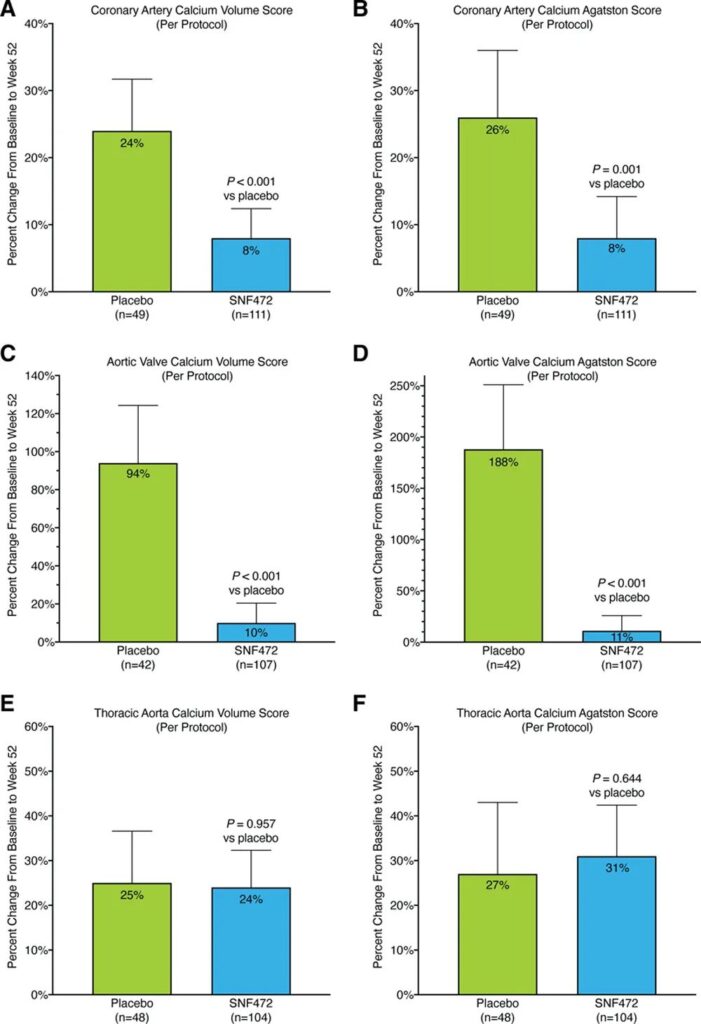
Secondary endpoint events include the log value of the coronary artery (CAC) Agatston score from 0 to 52 weeks; the log value of the aortic valve and thoracic aortic volumes core/Agatston score; and the patients whose coronary artery Agatston score progressed less than 15% at 52 weeks proportion
Combine the two groups of SNF472 and compare with the placebo group: the coronary artery and aortic valve calcification indexes (volumes core and Agatston score) of the SNF472 group are significantly reduced, and the thoracic aortic calcification is not obvious.
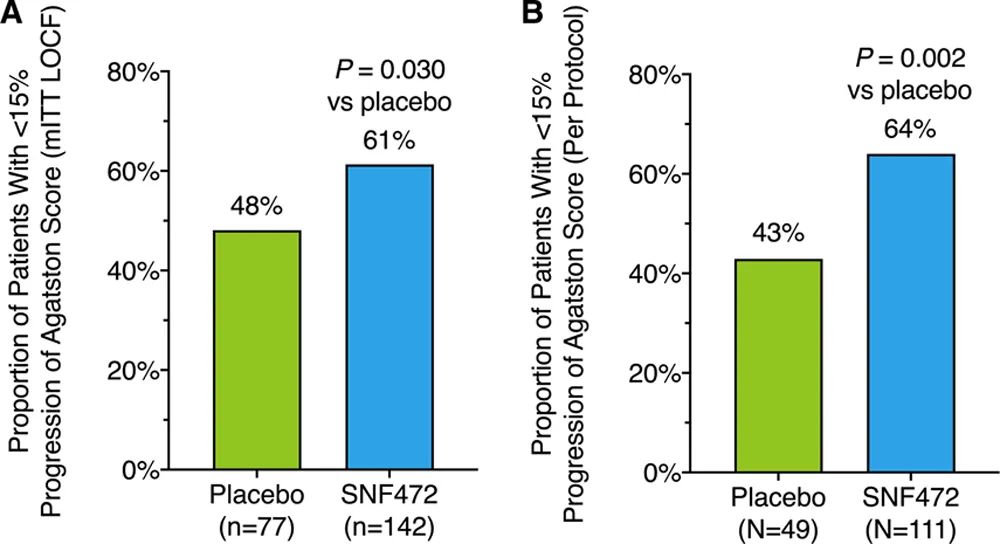
The proportion of patients with coronary artery Agatston score progression <15% at 52 weeks was 64% in the SNF472 group and 43% in the placebo group (p<0.05)
Adverse events (AE)
The three groups are basically similar

Evaluation
This is a phase IIb clinical trial with reasonable design and comprehensive follow-up
It should be noted that the loss rate of cases in the experiment reached 20% (15% in the placebo group, 16% in the low-dose group, and 29% in the high-dose group); in addition, ~60% of patients had received non-calcium phosphate adsorbents Therefore, the effect of SNF472 on vascular calcification is additional here; this trial did not show changes in hard indicators of cardiovascular events, which may be related to the follow-up time of only 52 weeks, and may be involved in subsequent studies.
Has the opportunity to target vascular calcification in ESRD patients been missed? In ESRD patients, is vascular calcification only a marker, not a treatable target? We need more and larger trials to answer these questions
(source:chinanet, reference only)
Disclaimer of medicaltrend.org



