History of IgG glycosylation and where we are now
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
History of IgG glycosylation and where we are now
History of IgG glycosylation and where we are now. IgG glycosylation is currently at the forefront of immunology and glycobiology, which may be partly due to the widespread and growing use of antibodies as drugs. The heterogeneity of galactosylation, fucosylation and sialylation has now become recognized factors, which can drive different functions of IgG, from inhibitory/anti-inflammatory to activating complement and promoting antibody-dependent cellular Cytotoxicity.
Therefore, if we want to truly understand how to design and deploy the most effective antibody drugs, and evaluate the correct vaccine response from the perspective of protection and function, we must have a deep understanding of IgG glycosylation.
IgG glycosylation structure
The function of IgG is driven by the crystallizable fragment (Fc) domain, which is encoded only by the gamma heavy chain and consists of two constant Ig domains, CH1 and CH2. Although this domain is not responsible for the antigen specificity of the Fab domain (including the variable region), it is the contact point for all FcγR molecules. In CH2, there is a generally conserved N-linked glycosylation site, asparagine 297 (N297).
This site mainly carries complex types of biantennary glycans, with different numbers of core fucose (Fuc), galactose (Gal), GlcNAc and terminal N-acetylneuraminic acid (Neu5Ac/sialic acid) residues ( figure 1). It helps maintain the Fc stability and the quaternary structure and function of IgG, and provides glycosylation modification sites for the binding of IgG to lectins.
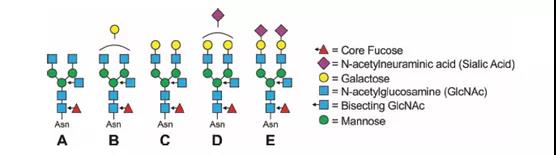
More importantly, these glycans fill a “big pocket” between the two copies of the CH2 domain, as shown in Figure 2.
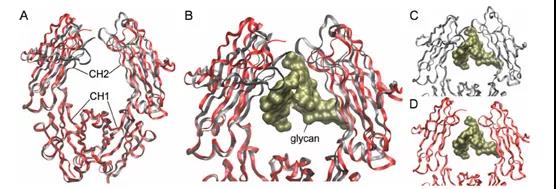
This article provides a detailed review of the literature on IgG glycosylation and clearly shows two overlapping eras. The first era began in the 1970s. During this period, the focus was mainly on the effects of inflammation and disease on IgG glycosylation and the functional impact of these changes.
In fact, most of what we know about the pro-inflammatory IgG glycotypes comes from this era, so these studies represent many basic principles that we know now. Inflammation is not only related to reduced galactosylation, increased fucosylation and bisecting GlcNAc, but also changes in Fc glycans also change the structural integrity and conformation of the Fc domain, as well as with Fcγ receptors (FcγRs ) Affinity.
Therefore, it was during this period that the relationship between IgG glycosylation, inflammation and function was largely mapped out.
Early stage
From 1970 to 2005, the focus was mainly on the effects of inflammation and disease on IgG glycosylation and the functional effects of these changes.
Galactosylation of RA
In 1976, Ciccimarra et al. found that four patients with variable gammaglobulinemia could not glycosylate their IgG heavy chains. In 1984, this finding was overturned.
In 1984, Dr. Thomas Rademacher and his team discovered that changes in IgG glycosylation are associated with rheumatoid and osteoarthritis (RA and OA, respectively). A number of subsequent studies laid the foundation for the idea that decreased IgG galactosylation is a sign of inflammation. .
In 1988, Pekelharing et al. RA was associated with the relative loss of galactose and the increase of terminal GlcNAc, while fucosylation was unchanged; and for the first time, it was shown that pregnancy not only transfers IgG glycosylation, but also transfers more galactose and Sialic acid may have an immunosuppressive effect, thereby temporarily limiting autoimmunity.
Galactosylation other than RA
In 1988, RA established an observation, observing that the degree of galactosylation of IgG lost with disease has expanded to include systemic lupus erythematosus (SLE) and Crohn’s disease. Mycobacterium tuberculosis, leprosy, and prostate cancer all found a corresponding decrease in galactosylation. Many conditions such as inflammation, infection, cancer or aging are related to the decrease of IgG galactosylation level.
The effect of glycans on function
In 1983, Dr. Hans Wigzel believed that glycans would actually change the function of IgG. In 1983, Nose and Wigzell found that the binding characteristics of antigen or protein A did not change, but they activated the ability of complement and the ability to induce antibodies related to FcγR on cells. Two years later, Heyman proposed that glycans can also participate in the promotion of anti-inflammatory function through FcγR.
In 1995, Adler et al. found that IgG lacking sialic acid or galactose was reported to have weaker binding to FcγR. In 2001, Mimura et al. found that removing galactose residues on Fc glycans did not change the binding to FcγRIIB, but removing GlcNAcs and mannose significantly reduced the binding. The above results indicate that IgG Fc glycans can differentially affect the binding of FcγR, and the results may depend on which receptors are present.
Glycan and Fc domain
In 1983, Sutton and Phillips showed that N297 glycans have powerful structural and functional effects on IgG. Krapp et al. (2003), Yamaguchi et al. (2006) and Mimura et al. (2000) confirmed that the structural integrity and thermal stability of the Fc domain depend on N-glycans.
1996 Lund et al. found that other mutations in the FcCH2 region lead to increased galactosylation and sialylation; α2,3- and α2,6-linked sialic acids are not the same in terms of Fc structure. In addition, the degree of sialylation seems to involve the end of glycan The accessibility and intramolecular interactions within the Fc domain are driven by the local protein conformation.
Translation glycomics
In 1994, Rademacher et al., by selectively separating IgG glycotypes lacking galactose into pathogenic antibodies, directly proved that specific glycotypes are not only related to diseases, but also can directly cause diseases.
IgG glycan and antigen immunity
In 1998, Parekh et al. studied that sudden exposure to new environmental/microbial antigens during the vaccination process would cause the same type of inflammatory transition in IgG glycosylation previously described in RA and other diseases.
New Era
2006-present The relationship between IgG glycosylation, inflammation and function is gradually being depicted
Turning Point (2006)
In 2006, Dr. Jeffrey Ravetch’s laboratory found that in the treatment of autoimmune diseases in the RA K/BxN model, IgGα2,6-sialylation is the main reason for the anti-inflammatory activity of IVIg/IgG.
In 2014, Chung et al. published an article showing that the increase in galactosylation and total sialylation can induce stronger antibody-dependent cellular phagocytosis (ADCP); in the same year, Quast et al. found that α2,6-IgG sialylation The complement-mediated cytotoxicity (CDC) is reduced, and the α2,6-sialylation of IgG1 has no significant effect on ADCC activity.
Core fucose and ADCC
In 2004, Okazaki et al. found that IgG molecules lacking fucose enhanced ADCC by increasing the binding and signal transduction of FcγRIIIA;
In 2011, Mizushima et al. found that the main reason may be that the core fucose causes steric hindrance at the interaction point, thereby reducing the binding affinity between the Fc domain and FcγRIIIA.
Towards high throughput
Relying on the HILIC method, in 2011, Pucic et al. found that in terms of trends, age was found to be the best predictor of the variance within the proportion of galactosylation, because the increase in galactosylation paralleled the increase in GlcNAc.
In 2013, Lauc et al. found that many trends in IgG glycosylation can be related to the results of the genome-wide association study (GWAS). The study identified four genes closely related to expected changes; they are ST6GAL1, B4GALT1, FUT8 and MGAT3. , They respectively encode the enzymes responsible for α2,6-linked sialylation, galactosylation, core fucosylation and bisecting GlcNAc addition in typical N glycans.
IgG glycans and regulation
In 2015, Wang et al. study showed that vaccinating patients with seasonal flu vaccine can cause glycoforms to shift over time. Studies in the following year showed that specific IgG glycotypes are permanently encoded in B cells and can be specifically induced, and that related glycotypes are optimized for the protective function required for this specific IgG target, but the explanation is controversial.
In 2016, Jones et al. IgGα2,6-sialylation was not controlled by IgG-secreting plasma cells, but it was sialylated after the release of ST6Gal1 circulating in the plasma.
The best predictor of variance within the lactosylation structure ratio, because the increase in galactosylation parallels the increase in the GlcNAc halving.
In 2013, Lauc et al. found that many trends in IgG glycosylation can be related to the results of the genome-wide association study (GWAS). The study identified four genes closely related to expected changes; they are ST6GAL1, B4GALT1, FUT8 and MGAT3. , They respectively encode the enzymes responsible for α2,6-linked sialylation, galactosylation, core fucosylation and bisecting GlcNAc addition in typical N glycans.
Summary and outlook:
Due to these profound differences in function, the concept of IgG glycosylation as a new method of disease treatment has rapidly matured. For example, Yamane-Ohnuki et al. deleted Fut8 (the fucosyltransferase responsible for core fucosylation). In this case, fucosylated anti-CD20 (target of rituximab) showed increased ADCC The use of sialylation to improve the efficacy of IVIg, first produced an IVIg, in which all IgG molecules are fully equipped with α2,6-sialylated glycans at N297. It is reported that a variety of animal In models (including collagen antibody-induced arthritis, K/BxN arthritis, ITP and epidermolysis bullosa), this drug candidate has 10 times higher anti-inflammatory activity than conventional IVIg.
This article provides a thorough historical perspective, combined with the latest information on IgG glycosylation, shows that the field of IgG glycosylation is broad and its impact cannot be underestimated. However, there are still many questions. Can the IgG glycotype be programmed, static or dynamic? Or is the truth somewhere in between? Will glycosylation engineering of antibody drugs improve efficacy? Can endogenous IgG glycans be directly manipulated as a therapeutic strategy in the body? Will this have unintended consequences?
This requires us to understand the impact of sugar chains on IgG, how their composition and structure are regulated, and how to manipulate them. The more capable the research community will eventually open the door to using sugar chains as the next frontier in medicine.
(source:ineternet, reference only)
Disclaimer of medicaltrend.org



