New antiviral drugs: Takeda maribavir Phase-3 trails success
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New antiviral drugs: Takeda maribavir Phase-3 trails success
New antiviral drugs: Takeda maribavir Phase-3 trails success. This will redefine the treatment of cytomegalovirus (CMV) infection/disease in transplant recipients!
Takeda recently announced the latest phase 3 data of the TAK-620-303 (SOLSTICE, NCT02931539) trial at the 2021 Transplant and Cell Therapy (TCT) Conference. The study is carried out in transplant recipients of refractory, with or without drug resistance (R/R) cytomegalovirus (CMV) infection/disease. The research antiviral drug TAK-620 (maribavir) will be combined with conventional antiviral drugs. Viral drugs (treatment specified by researchers [IAT], a combination of one or more of the following drugs: ganciclovir [ganciclovir], valganciclovir [valganciclovir], foscarnet [foscarnet], cidofovir [ cidofovir]) for comparison.
The primary endpoint of the study is the CMV viremia clearance rate confirmed at the 8th week of treatment (end of treatment period), and the key secondary endpoint is the CMV clearance rate and symptom control maintained until week 16.
The results show that maribavir has superior efficacy compared with conventional antiviral therapy (IAT), reaching the primary and key secondary endpoints of the study. In addition, maribavir has lower treatment-related toxicity than conventional antiviral treatments.
CMV is a DNA virus of the β herpesvirus subfamily, with a high degree of species specificity. Humans are the only host of human cytomegalovirus (HCMV). CMV is a common virus that can infect people of all ages. By the age of 40, more than half of adults have been infected with CMV, and most have no relevant symptoms and signs. However, in people with weakened immunity (including organ or stem cell transplant recipients), CMV infection is a serious clinical complication that can lead to tissue invasive disease and ultimately fatal. Existing antiviral therapies can be used to treat CMV, but these therapies may be limited in their application due to side effects and/or drug resistance.
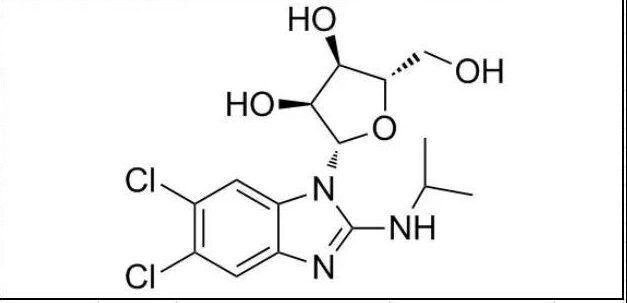
Maribavir is an orally bioavailable anti-cytomegalovirus (CMV) compound. It is currently the only one in phase 3 clinical development that is used in solid organ transplantation (SOT) or hematopoietic cell transplantation (HCT) for the treatment of post-transplantation Antiviral drugs for patients with CMV infection/disease. In China, maribavir obtained the implied license for clinical trials in April 2020, and its development indications are: for the treatment of cytomegalovirus (CMV) infection or disease.
Obi Umeh, MD, Vice President of Takeda and Head of the Maribavir Global Project, said: “We are excited about the results of the SOLSTICE trial. The detailed results shared at the TCT meeting in 2021 are an important development for transplant patients with increased CMV risk. If not controlled, CMV infection/disease may pose serious challenges. If maribavir is approved, it has the potential to redefine the treatment of refractory CMV after transplantation, regardless of resistance.”
The primary endpoint: In the eighth week of the study, among transplant recipients who received antiviral therapy for R/R CMV disease/infection, the proportion of patients who achieved confirmed CMV viremia clearance was in the maribavir treatment group (55.7%, n=131 /235) is more than twice the conventional treatment group (23.9%, n=28/117) (95%CI: 32.8%, 22.8-42.7; p<0.001). A subgroup analysis (random set) of the primary endpoint showed:
(1) Among the solid organ transplant (SOT) recipients who received antiviral therapy for R/R CMV infection/disease, 55.6% of the maribavir treatment group achieved confirmed CMV viremia clearance, while the conventional treatment group was 26.1%;
(2) Among hematopoietic cell transplant (HCT) recipients who received antiviral therapy for R/R CMV infection/disease, 55.9% of the maribavir treatment group achieved confirmed CMV viremia clearance, and 20.8% of the conventional treatment group;
(3) Regardless of the baseline viral load category (low load [<9100IU/mL], medium/high load [≥9100IU/mL]), among transplant recipients receiving antiviral therapy for R/R CMV infection/disease Compared with the conventional treatment group, a higher proportion of patients in the maribavir treatment group achieved confirmed CMV viremia clearance at week 8 (low load group: 62.1% vs 24.7%; medium/high load group: 43.9% vs 21.9%).
Key secondary endpoints: Data show that maribavir is superior to conventional antiviral drugs in eliminating CMV viremia and maintaining related symptom control until the 16th week. Analysis of key secondary endpoints (random set) showed that among the transplant recipients treated with maribavir, 18.7% (44/235) maintained CMV viremia clearance and symptom control at the 16th week of the study, while transplant recipients receiving conventional treatment Among them, 10.3% (12/117) (p=0.013).
In this study, transplant recipients treated with maribavir showed lower treatment-related toxicities, which are common in conventional antiviral therapy. Specifically, transplant recipients treated with maribavir had a lower incidence of treatment-related neutropenia compared with transplant recipients treated with valganciclovir/ganciclovir (1.7%[4/234 ] vs 25% [14/56]), compared with foscarnet-treated transplant recipients, the incidence of treatment-related acute kidney injury is lower (1.7% [4/234] vs 19.1 [9/47]).
The incidence of adverse events (TEAE) during any level of treatment in the maribavir group and the conventional treatment group were 97.4% (228/234) and 91.4% (106/116), respectively. The TEAE that led to discontinuation was 13.2% (31/234) in the maribavir group and 31.9% (37/116) in the conventional treatment group. There were 2 deaths due to treatment-related serious TEAEs (1 in each treatment group).
Francisco M. Marty, Associate Professor of Medicine at Harvard Medical School and Associate Physician at Brigham and Women’s Hospital, said: “We are very pleased that the SOLSTICE trial has reached the primary endpoint. Maribavir was compared with available antiviral treatments. Among patients treated with maribavir, more than half of the patients were able to successfully treat CMV infection within 8 weeks, and compared with currently available antiviral treatments (valganciclovir, respectively) /Ganciclovir and foscarnet) have fewer neutropenia and less acute kidney injury. These new findings are a major advance in the search for new treatments for cytomegalovirus for transplant recipients.”
Cytomegalovirus (CMV) is a beta herpes virus that usually infects humans; 40%-100% of the adult population has serological evidence of previous infection. However, individuals with compromised immune systems may develop serious illnesses, including patients receiving immunosuppressive agents associated with various transplants (including HCT or SOT).
CMV is usually latent and asymptomatic in the body, but it reactivates during immunosuppression. Among the estimated 200,000 adult transplants each year, CMV is one of the most common viral infections in transplant recipients. The estimated incidence in SOT transplant recipients is 16-56%, and the incidence in HCT transplant recipients is 30- 70%.
Reactivation of CMV can lead to serious consequences, including the loss of transplanted organs, and in extreme cases, it can be fatal. Existing therapies for treating CMV infection after transplantation may show toxicity, require dose adjustments, require hospitalization, or may not adequately inhibit viral replication.
Maribavir belongs to a class of drugs called benzimidazole nucleosides, which can target and inhibit the UL97 protein kinase of CMV, thereby potentially affecting several key processes of CMV replication, including viral DNA replication, viral gene expression, encapsidation, and mature coating The shell escapes from the nucleus of the infected cell.
Maribavir is an orally bioavailable antiviral therapy, currently in phase III clinical development, evaluating for hematopoietic stem cell transplantation (HSCT) or solid organs that are accompanied by CMV infection and are resistant or refractory to current standard CMV therapeutic drugs Therapeutic potential in transplant (SOT) recipients.
Currently, maribavir has not been approved by any country. In the United States and the European Union, maribavir has been granted Orphan Drug Designation (ODD) for the treatment of clinically severe CMV viremia in high-risk patient groups and for the treatment of CMV disease in immunocompromised patients.
In the United States, maribavir has also been granted breakthrough drug designation (BTD) for the treatment of CMV infection in transplant recipients.
(source:internet, reference only)
Disclaimer of medicaltrend.org



