Why mRNA COVID-19 vaccine can kick off earlier?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why mRNA COVID-19 vaccine can kick off earlier?
Why mRNA COVID-19 vaccine can kick off earlier? From the success of mRNA vaccines to the application of nano-pharmaceutical technology.
Both Pfizer and Moderna’s new coronavirus vaccines, which have been widely used, are based on new mRNA nanopharmaceutical technology. The mRNA fragments carrying the transcription information of the new coronavirus spike protein (S protein) are wrapped in lipids to make nanoparticles , Injected into the human body, using the body’s own cells to produce the S protein of the new coronavirus (S protein itself is harmless), which in turn stimulates the body’s cellular immunity (activating human T cells to recognize and engulf virus-infected cells) and humoral immunity ( Activate human B cells to produce specific antibodies that can neutralize viruses) and other immune mechanisms. Once the virus enters the body, this acquired immune response can protect us from infection.
Compared with traditional inactivated vaccines, mRNA vaccines are not only highly immunologically active, they also activate the human immune system without the intervention of virus particles or residual impurities during virus culture, inactivation, and purification; mRNA fragments are only transient in human cytoplasm It stays and cannot enter the cell nucleus, there is no possibility of changing the genetic material of human cell DNA, and no additional immune adjuvants are needed. Therefore, the mRNA vaccine is also safer.
The mRNA vaccine technology is the key to the perfect launch of these two vaccines in the United States due to its rapid design and construction, high resilience to virus mutation, as well as an efficient and versatile fully synthetic production process platform, and ease of standardized production. Factors, because these technical advantages meet the technical requirements for rapid R&D and large-scale, low-cost, and flexible production of emergency vaccines during outbreaks of infectious diseases. It is a favorable means for humans to fight against new infections in the future, with unlimited prospects.
The production of mRNA vaccines does not require large-scale cultivation of live viruses, and it also avoids the risk of virus leakage.
If the virus mutation causes the vaccine to fail, mRNA technology can also change the mRNA sequence in a short period of time (1-2 months), launch an upgraded version of the vaccine, and respond to the virus mutation in time.
Derived from the above advantages of mRNA vaccines, in the global COVID-19 vaccine research and development competition, compared with various vaccine technology routes such as inactivated vaccines, recombinant protein vaccines, viral vector vaccines, protein subunits, etc., it can be said that mRNA vaccines have won at the starting line. on.
1. The mRNA vaccine is new, but not unknown
In 1961, scientists successfully extracted mRNA for the first time, and then fully studied its function and biological behavior. Thirty years ago, a research team from the University of Wisconsin in the United States discovered for the first time that injecting in vitro transcribed mRNA into the skeletal muscle of mice can express the corresponding protein and produce an immune response. In the past two decades, with the development of mRNA synthesis, modification and delivery technology, mRNA technology has gradually become a weapon for biopharmaceutical companies to develop new products.
In recent years, scientists have studied mRNA vaccines against influenza, Zika virus, rabies and cytomegalovirus (CMV). Future mRNA vaccine technology may allow one vaccine to provide protection against multiple diseases, thereby reducing the number of vaccine injections. At the same time, mRNA technology has also been widely studied in the field of cancer prevention and treatment, and is considered to be a new hope for humans to overcome cancer.
The closest precedent to the mRNA COVID-19 vaccine is the nano-drug Patisiran that has been marketed by Alnylam Pharmaceuticals in the United States. This is a targeted siRNA transporting thyroxine protein for the treatment of amyloidosis. It was launched in the United States, the European Union, and Canada in 2018. And Japan was approved for listing. This new drug will transform the Nobel Prize-winning RNA interference research results into the first siRNA therapy for clinical use.
2. Where is the technical threshold for mRNA vaccines?
The immunogenicity of the mRNA vaccine and the stability of the preparation are the two major technical thresholds for its clinical application.
First, the mRNA chain in the vaccine may cause an unexpected immune response. In 2005, researchers from the National Institutes of Health (NIH) and the University of Pennsylvania successfully solved this problem by modifying mRNA technology. Each chain of mRNA is composed of four nucleoside molecules. They replaced one of them with a modified nucleoside to create a hybrid mRNA that can sneak into the cell without causing cell defense. Inflammation problems.
Secondly, mRNA has low permeability and high negative charge, and is easily degraded by RNase or cleared by immune cells. Therefore, it is difficult to obtain the desired effect in the body, and special preparation methods are required to make it commercialized. In 2015, American scientists made mRNA into Lipid Nanoparticles (LNPs) to improve stability and make it easier to enter cells, significantly improving the information transmission efficiency of mRNA vaccines.
These developments have made the widespread use of mRNA vaccines possible. Moderna (Moderna is the abbreviation of Modified RNA) is also a new company founded 10 years ago based on these new technologies. The approval of the mRNA vaccine will surely be a leap in the field of pharmacy, and it will be one of the most cutting-edge therapies in modern medicine. Therapeutic methods based on mRNA technology may be applied to other important fields at a faster speed, especially cancer treatment.
3. Application of Nano Pharmaceutical Technology
Lipid nanoparticles have a high degree of biocompatibility and biodegradability, with few adverse immune reactions. The most significant feature of nanomedicine is the high surface area to volume ratio, which enables efficient drug packaging. The encapsulated drug is protected from degradation and immune clearance, and due to effective drug packaging, the administered dose can be greatly reduced.
Nanopharmaceutical technology can greatly improve the safety and efficacy of drugs. It can deliver drugs through targeted drugs that bind to disease-specific receptors, and can add a second drug or even contain it in a different compartment. The desired release kinetics is administered. The success of the nano-COVID-19 vaccine has laid a solid foundation for the development of complex and non-lipid nano-drugs in the future.
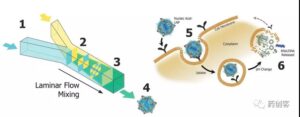
Lipid nanoparticles (LNPs) formulation Lipid nanoparticles (LNPs) formulation and its working principle (taking NanoAssemblr® technology platform as an example):
1) Inject the lipid organic solvent and the nucleic acid aqueous solution into the two channels.
2) Under laminar flow, the microscopic design in the channel causes the two fluids to mix in a controlled and reproducible way.
3) Within one millisecond, the two fluids are completely mixed, causing the polarity of the solvent to change, thereby triggering the self-assembly of the lipid nanoparticles loaded with nucleic acid.
4) Changing the speed and ratio of fluid injection can control the size of lipid nanoparticles.
5) Lipid nanoparticles mimic low-density lipoproteins so that they can be absorbed by endogenous cellular transport pathways to deliver nucleic acids to cells.
6) Due to the use of pH-sensitive lipids, lipid nanoparticles are allowed to release the encapsulated nucleic acid into the cytoplasm due to the decrease in pH and play a role.
The lipid components used to make LNP may cause allergic reactions in a small number of people. The severe allergic reactions caused by the two current mRNA vaccines in the United States are believed to be caused by these lipid components. CDC calculated the severe allergic reaction rate based on the observation results of 22 million people who were first vaccinated in the United States:
The incidence of Pfizer vaccine (50 cases occurred) is 5 parts per million (50/9,943,247)
- 94% (47/50) are women
- 90% (45/50) occurred within 30 minutes after injection
- 80% (40/50) have a history of allergies
The incidence of Moderna vaccine (21 cases occurred) was 2.8 parts per million (21/7,581,429)
- 100% (21/21) are female
- 90% (19/21) occurred within 30 minutes after injection
- 86% (18/21) have a history of allergies
All people who experienced allergic reactions were successfully treated.
Conclusion
- The platform advantage of mRNA vaccine technology meets the technical requirements for rapid development and large-scale production of emergency vaccines, and becomes an effective means for humans to fight against new infections in the future.
- The mRNA vaccine is highly immune and can stimulate multiple immune mechanisms such as humoral immunity and cellular immunity, and it also has higher safety.
- Nano-pharmaceutical technology is the basis for successful preparation of mRNA vaccines, and its application prospects are broad
(source:internet, reference only)
Disclaimer of medicaltrend.org



