Five new cancer drugs and new technologies may increase long-term survival!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Five new cancer drugs and new technologies may increase long-term survival!
Five new cancer drugs and new technologies may increase long-term survival! Conquering advanced solid tumors is not a dream!
The most notable progress in the field of tumor therapy is targeted therapy and immunotherapy, which provide new options for prolonging survival of patients with advanced solid tumors.
Among them, targeted therapy can be said to accurately kill cancer cells without damaging other normal cells in the human body. This is a treatment based on the cellular and molecular level, which can be understood as the “gene” drug. It can recognize the unique gene mutations of tumor cells, and use different targeted drugs to block the signal transduction pathways of tumor cell growth and reproduction for the identified carcinogenic sites, thereby killing cancer cells.
The immunotherapy, called the “fourth therapy”, is an absolute hot spot in the field of tumor treatment research at home and abroad, and it can be called a revolutionary breakthrough. As early as 2013, in the annual ranking of the top ten scientific breakthroughs by the “Science” magazine, immunotherapy was already at the top of the list. Both PD-1/PD-L1 therapy and numerous cellular immunotherapies have sprung up and developed rapidly, which undoubtedly set off an upsurge in cancer treatment.
So today, we will summarize the most popular anti-cancer drugs and cellular immunotherapy for the treatment of solid tumors. I hope that there will always be one suitable for cancer friends!
Drugs: Effective rate of 79%, not limited to the cancer type larotinib to overcome NTRK solid tumors
The latest data on the treatment of NTRK solid tumors with the broad-spectrum anticancer drug larotinib was updated at the 2020 American Association for Cancer Research (AACR) virtual annual meeting.
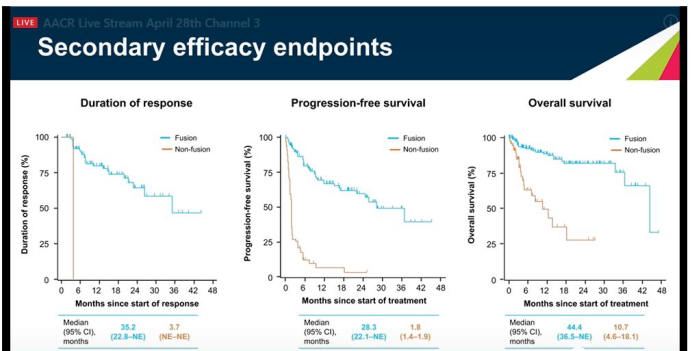
Among them, the median duration of response (DOR) in the NTRK fusion group was 35.2 months, and the DOR in the non-fusion group was 3.7 months. The median progression-free survival (PFS) of the fusion group was 28.3 months, and the median PFS of the non-fusion group was 1.8 months. The median overall survival (OS) of the fusion group was 44.4 months, and the median OS of the non-fusion group was 10.7 months. The fusion group was better than the non-fusion group in terms of median DOR, median PFS and median OS performance.
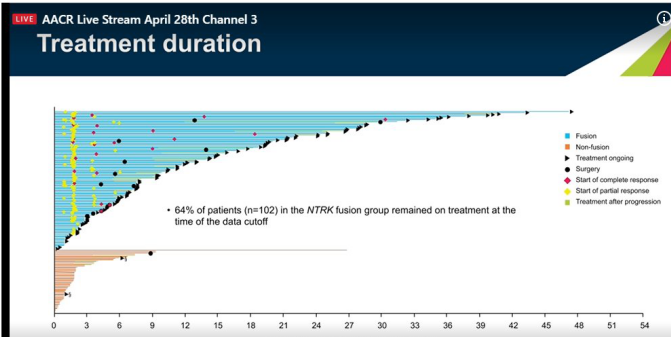
Treatment response: 64% of patients in the NTRK fusion group are still continuing treatment.
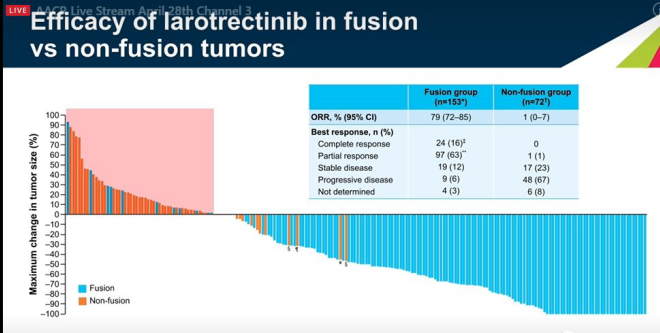
In terms of tumor size changes, the fusion group showed a larger reduction in tumor size compared to the non-fusion group. In terms of ORR, the ORR of the fusion group was 79%, and the ORR of the non-fusion group was 1%. The curative effect of the fusion group was significantly better than that of the non-fusion group. In terms of the best response, the CR rate in the fusion group was 24%, while in the non-fusion group, the CR rate was 0. It can be seen that the fusion group performed significantly better than the non-fusion group in terms of efficacy and best response.
Across the top ten cancer types! Highly effective anti-brain transfer target drug Entratinib is unstoppable
In August 2019, the FDA accelerated the approval of Entrectinib (Rozlytrek, Entrectinib, RXDX-101), the world’s third broad-spectrum “curing” anticancer drug, for the treatment of neurotrophic myosin in adults and children Receptor kinase (NTRK) fusion-positive, locally advanced or metastatic solid tumor progression after initial treatment, or solid tumor patients without standard treatment, and ROS1-positive non-small cell lung cancer (NSCLC) patients.
Clinical data shows that the response rate of pediatric tumor types carrying neurotrophic tyrosine receptor kinase (NTRK), ROS1 or anaplastic lymphoma kinase (ALK) fusion to treatment is as high as 100% (complete remission and partial remission)! Including intractable tumors in the central nervous system! This number is unprecedented, and it is believed to open a new door of hope for childhood cancer patients.
1) Among patients with NTRK fusion-positive solid tumors, the objective response rate ORR (tumor reduction) of entrectinib (entrectinib, RXDX-101) was 57.4%, and objective responses were observed across 10 different types of tumors (The tumor shrinks). Among patients with brain metastases, the ORR of entrectinib’s objective intracranial response rate was 54.5%, of which more than 1/4 achieved complete remission (all lesions disappeared).
2) In patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer NSCLC, clinical trial results showed that in 51 ROS1-positive NSCLC patients, the total remission rate reached
78%, The complete remission rate reaches 5.9%.
Disease control rate is 100%! BLU-667 recruits solid tumors
In May 2020, the New Drug Marketing Application (NDA) and Marketing Authorization Application (MAA) of Pralsetinib (BLU-667, Presitinib) for the treatment of locally advanced or metastatic RET fusion-positive non-small cell lung cancer have been approved by the United States. FDA and European Medicines Agency (EMA) accept. At present, pralsetinib has been granted priority review by the US FDA, and it is expected to make an approval decision before November 23, 2020. This means that if all goes well, in the fourth quarter of this year, we will usher in the second RET inhibitor after LOXO-292.
On July 2, 2020, pralsetinib (BLU-667, Presitinib) once again submitted a new drug application to the FDA for the approval of new indications for the treatment of patients with advanced or metastatic RET mutant medullary thyroid cancer and Patients with RET fusion-positive thyroid cancer. We look forward to the approval of this drug for marketing as soon as possible.
Technology: TILs therapy accurately kills tumor cells
Speaking of immunotherapy, everyone is familiar with PD-1 and CAR-T, but these are just the tip of the iceberg.
In the early stages of cancer, the immune system tries to attack the tumor by mobilizing special immune cells of lymphocytes. Lymphocytes have the ability to recognize and attack tumor flow and penetrate deep into the tumor. These cells are called tumor-infiltrating lymphocytes (TIL), which were discovered by Rosenberg and his team, the leader of the immune world.
Dr. Rosenberg believes that they are the most powerful immune cells that penetrate into the enemy’s interior, but due to some reasons (such as tumor microenvironment and PD-1), their functions are inhibited and cannot effectively kill tumors in tumor tissues. cell. However, scientists use some in vitro culture methods to enrich certain types of lymphocytes in these tumor tissues, and then return them to patients, which can play an anti-tumor effect, and the combined PD-1 effect will be better.
The first solid tumor cell immunotherapy won the FDA breakthrough therapy title
In June 2019, the FDA approved the tumor-infiltrating lymphocyte (TIL) treatment method LN-145 as a breakthrough treatment designation. This is the first time that a cellular immunotherapy for solid tumors has won this award. I believe that it is only a matter of time before it goes to market. Once approved by the FDA, this will be the first cellular immunotherapy for solid tumors and will bring huge survival benefits to cancer patients.
The FDA grant is based on the data from the ongoing second phase of the innovaTIL-04 (C-145-04) positive trial. The summary data shows that the overall response rate (ORR) for TIL treatment in patients with advanced cervical cancer is 44%.
As of the data cutoff on February 4, 2019, there were 27 evaluable patients.
The results show that:
1. 44% (12) of the patients had an effect, including 1 complete response, 9 partial responses and 2 unconfirmed partial responses;
2. The disease control rate is 89%;
3. The median follow-up time was 3.5 months, and 11 out of 12 patients responded continuously;
4. No serious side effects occurred.
5. The disease control rate is 89%! The first cellular immunotherapy to combat solid tumors won the FDA breakthrough therapy title!
Sum up:
In recent years, scientists have made considerable efforts to develop new drugs and new immunotherapies to overcome different cancers. We look forward to the fact that more and more pre-clinical/clinical trial data can piece together a complete puzzle, fully demonstrating the true strength of targeted drugs, PD-1/PD-L1 and cellular immunotherapy in treating many cancer types.
Five new cancer drugs and new technologies may increase long-term survival
Five new cancer drugs and new technologies may increase long-term survival
(source:internet, reference only)
Disclaimer of medicaltrend.org



