The first male contraceptive needle hasn’t been approved in 30 years?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The first male contraceptive needle hasn’t been approved in 30 years?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
The first male contraceptive needle hasn’t been approved in 30 years?
The first male contraceptive needle hasn’t been approved in 30 years? The first male contraceptive needle in the world, one needle tube has been used for 13 years, why hasn’t it been approved in 30 years?
In popular perception, contraceptives seem to be used only by women.
At present, there are more than 10 effective contraceptive methods for women, including oral contraceptives, patches, intrauterine devices, and contraceptive rings.
Among them, oral contraceptives have occupied a huge market share.
According to the statistics of Medicine.com, the size of the contraceptive retail market in 2017 was about 329 million US dollar, of which the emergency contraceptive market had the largest market share, accounting for more than 70%, and the contraceptive use rate reached 98.62%.
Among the dozens or even hundreds of contraceptives known, without exception, they are only suitable for women.
In contrast, the only contraceptive methods available to men are condoms and ligation.
According to a 2011 retrospective study, men who use condoms have an over 18% chance of making women pregnant.
Even if it is used according to the required standards, there is at least 2% of the possibility of contraceptive failure.
At the same time, because it can cause a decline in sexual experience, in male groups, condom use compliance is poor. The ligation is even more irreversible because the operation is “a fix for life” and almost no one cares about it.
In 2002, a survey of 9,000 men from four continents showed that 55% of men said they would use safe and reliable male contraceptives if there is a suitable one.
The huge market demand and the limited choice of contraceptive methods have made men who “make frequent mistakes” in the relationship between the sexes expect a contraceptive method that is comfortable, effective, and non-invasive.
But the reality is that from the beginning of the research and development in the 1950s to the present, there is no reliable contraceptive pill that can be accepted by men.
“Although the media periodically come out to predict that new male contraceptives will appear in 5-10 years,” said John Amory, a professor at the University of Washington School of Medicine, “but the funny thing is that we have been saying that for the past 30 years. “
Male contraceptives seem to have reached a bottleneck until it appears.
Changing the emergence of the male contraceptive industry: RISUG
In the 1980s, due to the rapid population growth, a male contraceptive called RISUG (Reversible Inhibition of Sperm Under Guidance) was born in India.
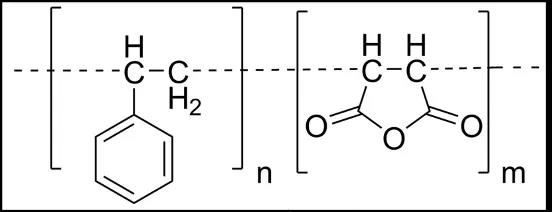
While studying the purification of the sewage pipeline system, researchers accidentally discovered that a polymer material-styrene maleic anhydride (SMA) can be lining the pipeline, killing bacteria without corroding the pipeline itself, and disinfecting the pipeline of the pivot pump. effect.
SMA, also known as styrene maleic anhydride, is a synthetic polymer formed from styrene and maleic anhydride.
It is widely used because of its high heat resistance, stability and strong reactive acid anhydride groups. In the field of engineering plastics. At the same time, SMA has extremely high solubility in dimethyl sulfoxide (DMSO).
However, the good times did not last long. Due to the interruption of cooperation, the research work of the scientific researchers on the SMA purification sewer system had to be terminated early.
However, there is no SMA used in urban sewage pipes. Instead, it is applied to another type of pipe in the male body-the vas deferens.
The vas deferens is a pair of curved thin tubes, one end communicates with the epididymal duct, and the other end merges with the seminal vesicle gland.
The seminal vesicle gland sends out the ejaculatory duct and then opens into the posterior urethra.
Its main function can transport sperm from the epididymis to the urethra, which is the only channel for mature sperm to be transported from the epididymis to the urethra of the prostate.
After years of painstaking research, a new male contraceptive pill, RISUG, was finally developed.
It is a composite polymer obtained by dissolving SMA in proportion to DMSO. According to its characteristics, researchers have devised a method of contraception that does not require invasive surgery.
The principle of operation is very simple. The doctor will first make a small opening in the scrotum, pull out the vas deferens, and inject RISUG into the tube.
It can rely on DMSO to attach to the inner wall of the vas deferens and form a thin film within 72 hours.
The film does not completely prevent normal sperm from passing through the vas deferens, avoiding a situation similar to the excessive pressure in the epididymal tube after vasectomy.
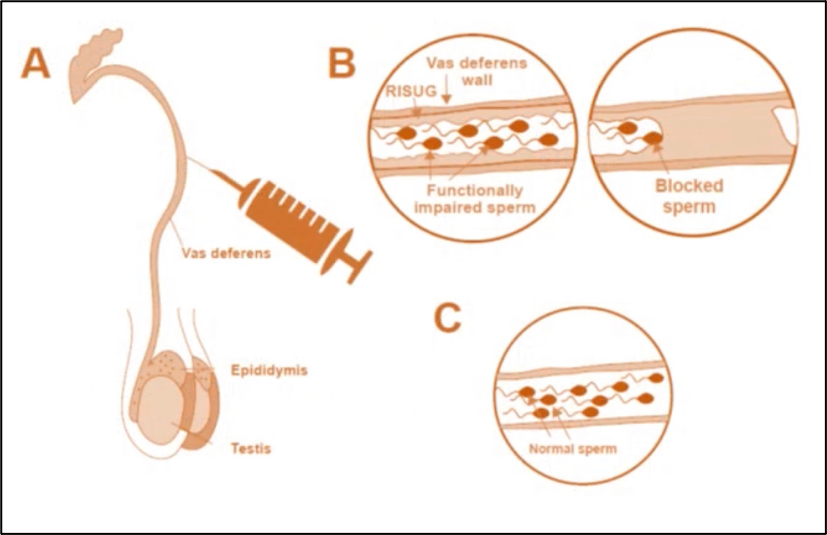
RISUG mixed polymer mainly achieves contraceptive effect through two aspects.
The formed film can hinder the outflow of sperm to a certain extent, but this does not produce a direct contraceptive effect.
The most important thing to achieve contraception is to “electrode” sperm.
The COOH group of SMA exposed in the lumen can ionize hydrogen ions. By attracting the negatively charged protein in the tube fluid, the positively charged side of the protein is turned toward the lumen, forming a huge positive charge within 48 hours “Protein Set”.
When the negatively charged normal sperm pass by, the positron effect of the “protein sheath” can cause damage to the sperm acrosome; at the same time, the positron can create an acidic environment, so that the sperm is destroyed before it rushes out of the vas deferens, thus achieving contraception purpose.
In addition, RISUG will not permanently affect male reproductive function. When a drug user decides to restore fertility, SMA can be completely dissolved by injecting DMSO, and the polymer that loses SMA quickly loses its “spermicidal” effect.
Due to the good experimental results in mice and monkeys, the clinical trials of RISUG began to be carried out in an orderly manner since the 1980s.
In 1993, researchers carried out the first phase I clinical trial, which included 38 adult normal men.
They injected different concentrations of SMA mixed polymer to verify the safety of the drug.
The test results show that the aggregates containing only DMSO or low-dose SMA have little long-term effect on sperm count, but when SMA exceeds 60mg, it can significantly reduce sperm count.
In addition, the drug has no effect on sexual desire, and all test subjects indicated during the follow-up that they can ensure a normal frequency of sexual life after using the drug.
Subsequent phase II and phase III clinical trials have given people hope for their super-high effectiveness and excellent safety.
From 1998 to 2017, at least four phase III clinical trials were completed, with an average effective rate of 97.7% and the highest effective rate of over 99%.
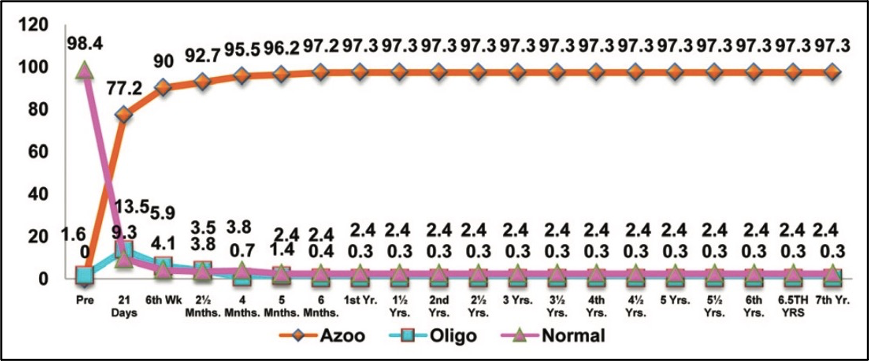
In addition, the safety of RISUG is also recognized. The follow-up time for completed clinical trials ranged from 6 months to 7 years, and no serious side effects were reported.
Ronald Weiss, Canada’s top urological surgeon, after visiting India with the WHO to learn about RISUG, said, “This method has extremely high application prospects. If its effectiveness and reversibility can be proven, then vasectomy will be impossible. People are interested.”
What does RISUG mean for male contraceptives?
In fact, RISUG is not the first male contraceptive.
Since the discovery of the anti-sperm effect of gossypol in the last century, the exploration of male contraceptives has never stopped, and related clinical work has sprung up like bamboo shoots after a rain.
But because long-term use will cause side effects such as hypokalemia and even permanent azoospermia, gossypol gradually fades out of sight.
The male contraceptives explored later are roughly divided into two categories: one is hormonal contraceptives, and the other is non-hormonal contraceptives.
The former is through the administration of exogenous male hormones, negative feedback inhibits the male endocrine axis, and ultimately inhibits the synthesis of endogenous testosterone and sperm formation; the latter mainly directly affects sperm production or indirectly inhibits sperm motility and other non-germ cell activities, and finally achieves contraception effect.
However, regardless of the contraceptives in the two categories, due to the limitations of the drugs themselves, they have stopped in the first and second phase clinical trials.
In summary, the following factors have contributed to its development dilemma.
First of all, the success rate of drugs is difficult to guarantee.
Unlike men, women’s ovulation cycle can be as accurate as a clock and can easily be adjusted by hormone drugs to achieve contraceptive effects.
Moreover, the number of women’s ovulation each time is limited, and the use of drugs is conducive to control, and the effectiveness is high.
However, due to the difference in natural physiological structure, contraceptive pills are like a goalkeeper on the court defending hundreds of millions of goals at the same time.
It is no less difficult than letting a child under the full moon save consecutive “Messi + Ronaldo” fixed-point shots.
The difficulty can be imagined. Most hormonal drugs are “medium collapse” because of their poor effectiveness and short duration.
In addition, some drugs cannot restore fertility after stopping. Generally speaking, safe contraceptives should be guaranteed to restore fertility within 16 weeks of stopping the drug.
However, non-hormonal drugs such as gossypol that directly inhibit spermatogenesis are difficult to reverse fertility in a short time, which has become one of the main difficulties in ensuring the safety of male contraceptives.
In addition, the side effects of drugs also make development difficult. For contraceptives used externally, large doses are often required.
In addition to possible weight gain and reduced immunity, it also reduces the content of high-density lipoprotein in the blood and increases the risk of diseases such as atherosclerosis.
Compared with various male contraceptives that have appeared before, RISUG is currently the only male contraceptive that has completed Phase III clinical trials.
With its high efficiency, recognized safety, and reversibility that reassure men, it seems that RISUG can really change the dilemma of male contraceptives.
Why is it that it is effective but not listed for a long time?
As early as the end of the twentieth century, when the first phase III clinical trial was completed, WHO sent a working group to analyze the safety and effectiveness of RISUG, and Ronald was among them.
However, the hopeful RISUG was poured cold water before it was born: WHO denied the possibility of its global promotion.
It turned out that after investigations, WHO found that the production of the drug only stayed at the “small workshop” stage and did not meet the mass production standards of the drug at all.
At the same time, due to the lack of some data, investigators pointed out that RISUG does not comply with the international new drug quasi-management regulations, safety and effectiveness cannot be guaranteed, and cannot be promoted worldwide.
Due to the passage of time, the original WHO RISUG analysis report has nowhere to be found. However, according to the development of Indian drugs, its doubts about the drug are actually not unreasonable.
According to current standards, in order to be approved by the US FDA, drug production must reach a certain scale of mass production.
According to an article published in 2018 that systematically compares the differences in new drug approvals between India and the United States, to obtain FDA approval, a production scale of at least 1 million doses is required.
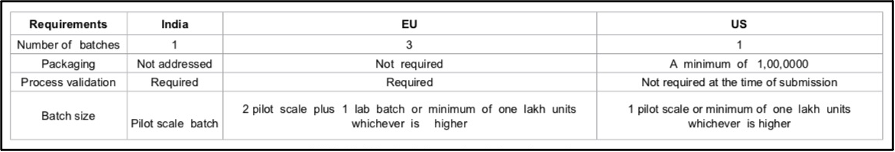
Source: Reference 10
In contrast, India has no production scale requirements. If the mass production requirements are not in place and only small-scale production remains, it will not be able to pass the FDA review.
In addition, compared with the WHO recommended guidelines, India is very irregular in the safety review of new drug approvals.
For example, when reviewing some drugs, the Indian Drug Administration does not personally review the results of clinical trials, but hands them to other drug companies to provide expert review opinions, and approve them based on the review opinions.
Even the current drug approval rules formulated in 1988 stipulate that in the case of “in the public interest”, the FDA may approve the use of drugs under the condition that the effectiveness and safety of the drugs still lack scientific evidence.
However, the Indian side insisted that the safety of the test is beyond doubt.
“We have completed clinical studies on 282 volunteers from five centers across the country, and no adverse reactions have occurred,” said Dr. Shama, director of the Reproductive Center of the Indian Institute of Medical Research. “During my 25-year research on the RISUG drug In, there has never been a problem report.”
“The current delays are all caused by the NIH in the United States,” a RISUG researcher said. “Even at the WHO, many people are unwilling to see drugs pass the drug review because they want to develop hormones that can have continuous demand and bring long-term benefits. Contraceptives”.
The reason behind all kinds of obstruction is simple: RISUG is unprofitable.
Compared with the existing male contraceptive technology, the cost of RISUG is extremely low. According to reports, the price of this drug is only $10. Compared to the billions of dollars in profits generated by the female contraceptive and condom industry each year, this is very small.
At the same time, RISUG has a long “standby” ability, and one injection can maintain the contraceptive effect for 13 years. For pharmaceutical companies, this “ten years at a time” curative effect has not attracted “repeat customers” at all.
Therefore, RISUG, which has extremely low use cost and long continuity, is naturally rejected by profit-oriented pharmaceutical companies.
Of course, not only did the Indian side consider the audit to be problematic, Dr Ronald also disagrees with the WHO’s conclusions, so since the late 1990s, he has worked hard to promote the landing of RISUG in North American countries.
However, when submitting materials to the Canadian Health Administration, I was told that all trials must be redone due to differences in clinical trial standards and ethical requirements. It would cost millions of dollars to rerun each experiment, which is almost impossible.
The ideally plump Ronald was rubbed on the ground by the skinny reality, and was finally forced to abandon the marketization of RISUG in North America because of the lack of research and development.
The two parties have been controversial over the safety and effectiveness of the drug for decades. But in any case, a veto by the WHO made RISUG, who had not yet gone abroad, sink into the sea.
At the same time, RISUG’s road to market in India has not been smooth. Since the Phase III clinical trial in the 1990s proved its effectiveness, in order to further expand the phase III trial scale to obtain higher evidence-based medical evidence, the Indian Research Institute Staff began to recruit more volunteers to participate in clinical trials.
However, in the past two years, only 64 volunteers participated, which is far from reaching the 500 volunteers recruiting target of the Medical Committee of the Ministry of Health of India.
The limitation of ideas has played an important role in it, and it is the men themselves who really hinder the listing of this drug.
India is a patriarchal society, and the status of women in the family is much lower than that of men. In the stereotype, men generally believe that pregnancy, childbirth, and family life are entirely women’s responsibilities and have nothing to do with them.
This creates a major obstacle to the promotion of RISUG in India-Indian men will naturally think that contraception is not their own business.
RISUG, which was originally walking on the “Bright Road”, seems to have come to an end. This male contraceptive pill that may change the world is waiting for someone to restore it.
Because of “equal rights,” she relied on her family property to save RISUG
“No woman can live to see a man using contraceptives during her childbearing period,” said Carl Djerassi, a top chemist known as the “father of female contraceptives,” in a Stanford University class. “After so many years, all male contraceptives are still in the developmental stage.”
The whole class burst into laughter, except for Elaine Lissner, who was still in graduate school at the time, because her brain repeated a question, “Why do women have to bear huge contraceptive measures? Why are so many women choosing No male choice?”
With the purpose of changing the status quo of contraception in the world and voicing for “affirmative”, in 2005, she established a non-profit organization called the Male Contraceptive Information Project (MCIP) to promote the research and development of male contraceptives worldwide.
Later, she discovered that RISUG is a promising drug candidate. However, due to lack of funds, she can only stay at tracking and publishing relevant research information, and cannot play a substantial role in the development of new drugs.
In 2009, with the rise of the real estate industry in the United States, Irene, who invested in real estate owned by her father in the early days, made a lot of money in a short period of time. She quickly used the money she earned to establish a private fund, Parsemus, and began to support RISUG’s drug development.
In February 2010, Parsemus bought the international authority of RISUG for US$100,000 and launched international cooperation with India to research and develop RISUG.
When originally preparing to fight the ambition and promote the launch of RISUG in the United States, Irene was told by the FDA the same requirements as Ronald “If we want to pass, we need to re-launch all basic and clinical trials.”
This means that Irene needs at least US$500,000 to carry out clinical trials, and the entire approval process needs US$4 to 5 million.
But she did not give up. In order to avoid confusion with RISUG in India, she decided to rename Vasalgel, adjust the original drug formula, and redo all trials in the United States.

Source: YouTube screenshot
According to the official website, the research team under Parsemus has been conducting basic toxicology tests since the end of 2011.
At present, its animal safety has been guaranteed and comprehensive clinical trials have begun.
In 2011, with the help of Irene, the Indian research team received a $100,000 Gates fund. Although $100,000 is a drop in the bucket for new drug research and development, it brings the most important thing to RISUG: attention and acceptance.
According to more than Parsemus websites, more than 23,000 men and women have signed agreements and are willing to participate in Vasalgel’s clinical trials.
In addition, the research and development of the drug has received full support from the government.
The Indian government plans to recruit 500 more patients nationwide to conduct multiple clinical trials.
“I will keep improving the safety and effectiveness of the drug, so that no one will worry about its side effects. Our goal is like a condom advertising line.” Irene mentioned in the interview, “It’s so good that you completely forgot about it.” it.”
Irene, 52, said in an interview that she would not give up the development of Vasalgel.
“Reproductive rights, women’s health, male rights, environmental population, unwanted pregnancy, these issues are actually tightly tied together.
If people can freely decide when to have children, it will solve the problems faced by human beings, including poverty. A series of questions create unlimited possibilities.”
According to the official website, Vasalgel has completed animal efficacy and safety tests, and is currently conducting phase I clinical studies in an orderly manner.

Source: Vasalgel official website
In India, after years of clinical trials and research, RISUG researchers once again submitted a drug marketing application to the Indian Drug Administration, and the review results are expected to be announced next year.
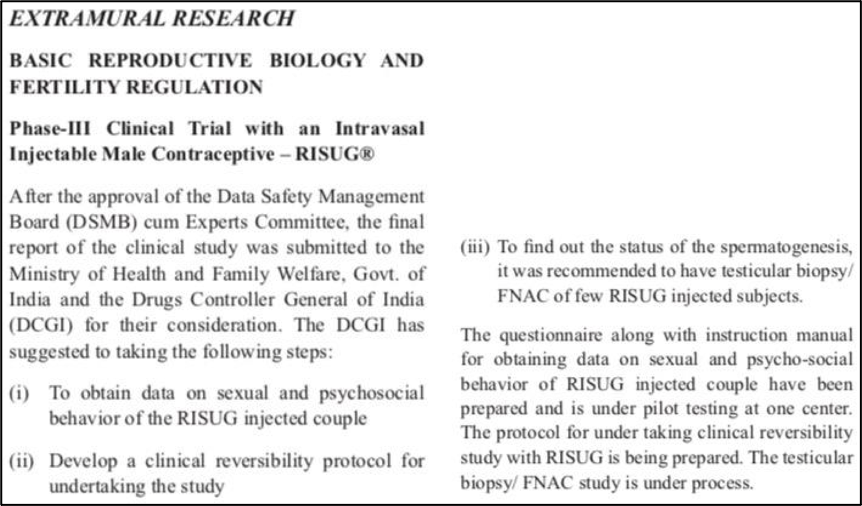
Source: Reference 9
Currently, there are more than 213 million pregnancies worldwide each year, of which 40% (85 million) are unintended pregnancies, and 50% (106 million) ultimately choose abortion.
If RISUG or Vasalgel is approved by the National Food and Drug Administration, it will be the first male contraceptive in human history since the condom was invented 400 years ago.
The first male contraceptive needle hasn’t been approved in 30 years
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



