Use PAT in CART cell production process
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Use PAT in CART cell production process
Use PAT in CART cell production process. Let’s talk about PAT first. In order to ensure the realization of cGMP, FDA issued the PAT white paper “PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance” in 2004. The white paper mentions that the promotion strategy of PAT is to enable enterprises to pass the review of CMC (chemistry manufacturing and control) and cGMP through PAT.
Process Analytical Technology (PAT) is defined as “a system that designs, analyzes, and controls manufacturing through the timely measurement of starting materials, process materials, and key quality and performance characteristics of the process during the production process, and to ensure the quality of the finished product. “.
Process Analytical Technology (PAT) can measure raw materials, intermediates, and products. Measurements can provide important data to understand how process variables affect chemical, biological processes or particle-based systems. PAT provides an opportunity to measure previously unknown intermediates, mechanisms and endpoints, and can be used in R&D, scale-up production and manufacturing processes.
1 Multivariate analysis tools
From the perspective of physics, chemistry or biology, chemical or biochemical processes are complex multi-factor systems. Multivariate Data Analysis (MVDA) is the basis for understanding and mastering the entire system. To ensure the adaptability of modeling and the reliability of prediction, a statistical approach is usually adopted. If used properly, multivariate analysis tools can not only identify and estimate the variables that determine product quality and performance in the product and process, but also identify potential failure modes and mechanisms, and quantify the factors affecting product quality.
2 Modern process analysis instruments
Process analysis instruments are not as simple as installing offline instruments directly into the production process. The design and manufacturing of instruments and their interfaces with the process should first consider that the collected data must be related to the process and product quality and reflect its characteristics; In the design, the durability, reliability, and ease of operation of the instrument should also be considered; in addition, the installation of the instrument requires risk analysis, and it should not cause damage to production operations and product quality. Compared with offline measurement, online instruments and meters have more restrictions. At present, there are no recommended online instruments and meters in CART production.
3 Process control tools
By closely linking product design and process development, feasible and effective process control strategies can be formulated. Through monitoring of the process status, the process can be maintained in a set state, which fundamentally ensures that the key product quality can be effectively controlled. The control strategy should be able to adapt to changes in the input of raw materials, the ability and reliability of process analysis instruments to measure key attributes, and the endpoint judgment to ensure consistent product quality.
PAT does not determine the end of the process at a certain fixed time, but determines whether the target product meets the quality requirements, that is, the process control strategy should be formulated and adjusted based on the actual monitoring of the product. But it is not that the process runs without considering the time, it is necessary to estimate the normal completion time of the entire process, and it also provides the maximum time deviation that may occur.
The PAT framework covers the entire production process. The online material and product evaluation work is much more than the work in the laboratory. The quality of the product needs to be determined based on strict statistical principles, and the advantages of multivariable statistical control will be fully utilized.
4 Continuous improvement and knowledge management tools
During the product life cycle, knowledge can be continuously discovered and accumulated through data collection and analysis, which will play a role in future calibration and adjustment. The method and information system for acquiring knowledge from the process database is of great significance to product production. The advantage of the knowledge base is to establish the corresponding relationship between knowledge and a variety of factors, and use this knowledge in different corresponding situations, such as establishing guidance and monitoring production process models.
From the perspective of the information architecture of process operation, PAT is under the execution layer of the process information system, namely the Manufacturing Execution System (MES) (as shown in the figure below). Compared with the traditional MES architecture, the introduction of PAT has greatly strengthened the process understanding of the MES, which is also the main purpose of the FDA to promote PAT.
Let’s take a look at the description in the document <BioengineeringSolutions for Manufacturing Challenges in CAR T Cells>:
According to the production process of CART cells, somatic cell therapy involves collecting cells through apheresis, then performing T cell activation, CAR gene transfer, T cell expansion, and quality control and assurance (QC/QA), and then injecting CAR T cells into the patient in vivo. It usually takes 22 days [15].
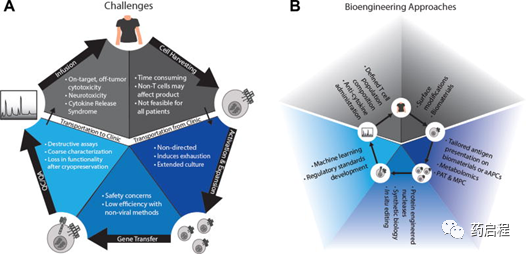
Challenges in the manufacturing process of CAR T and potential bioengineering solutions.
A) The manufacturing process of autologous CAR T cells. Autologous cell therapy involves collecting cells through apheresis, then performing T cell activation, CAR gene transfer, T cell expansion, and quality control and assurance (QC/QA), and then injecting CAR T cells into the patient. Each of these steps has many existing challenges that affect the safety, efficacy, and scale of CAR T cell production. B) Bioengineering methods to improve CAR T cell manufacturing.
Biological engineers can assist in the quality control and assurance of CAR T cell products by using process analysis technology (PAT) and model predictive control (MPC). MPC is a tool in which the workflow can be managed by mathematically predicting the results based on the current measurement state of the process, thereby significantly improving efficiency and automation [105] (Figure 3).
However, these techniques are rarely used in mammalian cell culture-based processes, [106] mainly due to the lack of monitoring tools. [107] Since the advanced process control technology of mammalian cell culture relies on metabolic flux analysis, studying the metabolic requirements of T cell subpopulations may yield useful monitoring targets [108,109].
PAT used for T cell culture may include immunological biosensors [110] and spectroscopy techniques [111]. Soft sensors can be used to integrate measurements of secreted cytokine and metabolite concentrations with software modeling to estimate other components [112]. In CAR T cell expansion, multiphoton redox-based imaging technology combined with biosensors can be used to detect intracellular respiration [75] to detect secreted cytokines [113], thereby potentially identifying the phenotypic distribution of T cells. As with biopharmaceuticals [114], it is expected that in the near future, regulatory agencies may require improvements in cell manufacturing, PAT and automation based on design quality to be integrated into the current CAR T cell production paradigm.
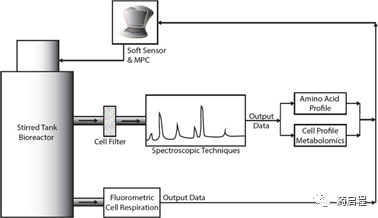
Process analysis technology (PAT) and model predictive control (MPC) implementation of CART cell population in the manufacturing process. An online spectrometer was used to sample the media from the bioreactor to determine the amino acid composition and metabolite concentration. Analyze cells from the bioreactor using fluorescence technology to determine their respiratory characteristics. Use the model to combine these outputs to estimate the cell composition in the bioreactor and adjust the media composition in-situ to optimize cell yield.
Figure 3 Process analysis technology (PAT) and model predictive control (MPC) implementation of CART cell population in the manufacturing process. An online spectrometer was used to sample the media from the bioreactor to determine the amino acid composition and metabolite concentration. Analyze cells from the bioreactor using fluorescence technology to determine their respiratory characteristics. Use the model to combine these outputs to estimate the cell composition in the bioreactor and adjust the media composition in-situ to optimize cell yield.
With advances in biomaterials, genome engineering, tissue engineering, metabolic engineering, process control and synthetic biology will lead to the production of more advanced CAR T cells to produce PAT, the production of this therapy can be more easily implemented in various medical fields .
(source:internet, reference only)
Disclaimer of medicaltrend.org



