Lentivirus production Process optimization and Suspension culture
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Lentivirus production Process optimization and Suspension culture
Lentivirus production Process optimization and Suspension culture. Lentiviral vectors can transduce both dividing cells and non-dividing cells.
They are considered safe and can provide long-term transgene expression. It is currently one of the most common gene transfer methods.
The success of chimeric antigen receptor (CAR) T cells in the treatment of B-cell leukemia and lymphoma is particularly striking. This approach relies heavily on lentivirus-mediated gene transfer.
The production of lentiviral vectors can be carried out in different ways, but the most common is according to the method of Merten et al. [11,12], which involves the use of mammalian cells in an adherent or suspension cell system.
The most widely used method for producing lentiviral vectors is to transiently transfect HEK293T cells with vectors containing expression, capsid (one or two plasmids) and envelope cassettes [13]. These cells are widely used because they are highly transfectable [14].
The HEK293 cell line derived from human embryonic kidney cells is a mature LV production system because they are very suitable for suspension culture and easy to transfect [5]. Suspension culture in serum-free medium has advantages in large-scale production because it eliminates batch-to-batch differences and reduces the risk of indeterminate factor contamination [5]. In order to solve the cytotoxicity problem caused by viral proteins (ie, Gag, Rev, VSV-G), the expression of these elements is usually regulated by an inducible promoter, which is only activated during production [6].
This article mainly introduces a typical program for producing lentivirus, concentrating by ultracentrifugation and determining virus titer. The resulting virus can then be used in a laboratory analysis method for gene transfer (flow cytometry detection method). Describes the method of transfection of adherent human cell lines and creates an optimized platform to produce lentiviral vectors for CAR-T cell applications. Three methods of LV production are introduced: transient transfection, induction of stable packaging cell lines and induction of stable production cell lines.
1. Introduce a scheme for small-scale production of lentivirus
Describes the production of a small volume of 10 mL lentivirus. This process can produce low-titer virus, usually about 1×106 transduction units (TU)/mL, which is suitable for tissue culture assays or the production of stable cell lines. If a large amount of virus is required (usually in the case of CAR T cells), a large amount of virus must be produced and then concentrated by ultracentrifugation. This step can increase the virus titer by 100-1000 times. Then it can be detected with a flow cytometer.
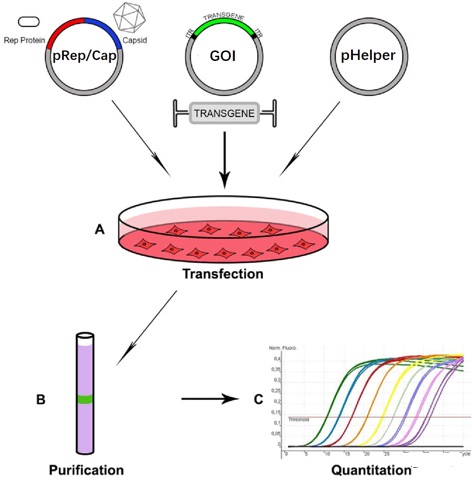
The small-scale cultivation parameters are as follows:
1. Keep the 293T cells in a humidified incubator at 37°C and 5% CO2. According to standard tissue culture procedures, it is very important to use trypsin to split 3 times a week. The medium used is DMEM 10% FBS (see Note 1).
2. Coat a 10 cm petri dish with 1 mL of 0.1% EIA gel solution, incubate at 4°C for at least 1 h, and then aspirate all the solution (see Note 2).
3. 24 hours before the start of the transfection protocol, 4×106 cells were seeded in a pre-treated 10 cm dish.
4. At least 1 hour before transfection, remove the used medium and replace it with 10 mL of fresh medium + chloroquine, and replace the culture dish in the incubator (see Note 3).
5. Mix the plasmids in FBS-free medium to make the final volume 500 μL. Plasmid used in this protocol: packaging plasmid pSPAX2 (9μg). Pseudotype envelope plasmid VSV-g (3μg). Vector plasmid FUGW (10μg).
6. Dilute PEI in 500 μL DMEM without FBS. Generally, for 1 μg DNA, 3 μL of 1 mg/mL PEI solution will be required. In this protocol, 66 μL of LPEI solution is added to 434 μL of FBS-free DMEM (see Note 4).
7. Add the diluted PEI dropwise to the test tube containing the plasmid mixture. Incubate at room temperature for 15-20 minutes.
8. Homogenize the mixture, and then gently drop it into the cells. Incubate overnight (18 hours).
9. Replace the medium with fresh DMEM 10% FBS.
After 10.48 hours, harvest the supernatant in a conical tube (see Note 5).
11. Centrifuge at 300 g for 5 minutes to pellet cell debris. Place the virus supernatant in a new conical tube.
12. Dispense into 1.5 mL micro test tubes and store at 80°C (see note 6).
Notes:
1. For successful lentivirus, low-passage 293T cell stock solution is the first choice. The use of these stock solutions can achieve a higher transfection rate, and therefore, increase the virus titer.
2. It is known that 293T cells are easy to fall off the plate. Using EIA gel will help fix the cells and avoid loss during the process [11].
3. Use chloroquine at this concentration to help 293T incorporate into the plasmid and express the transgene. It works by reducing autophagy, reducing plasmid degradation and promoting the escape of plasmid from endosomes.
4. Although general guidelines are used in the transfection protocol, the ratio of DNA:PEI should be determined empirically to optimize transfection. In short, different ratios should be used (for example: 1:2; 1:3; 1:4; 1:5; 1:6; 3:1; 3:1; 3:1; 3:1; 3: 1; 3:1; 3:1; 3:1; 3:1; 3:1; 3:1; 3:2; 3:1) and 4:1 (2:1) were transfected with control plasmids respectively. 1:7; 1:8) and analyze the transfection efficiency of the cells.
5. The harvest time point can be changed and optimized. For lentivirus, peak production is reached between 48 and 72 hours. The supernatant can also be harvested and replaced with fresh medium, allowing the second harvest to be collected the next day.
6. Generally, small portions are used to store lentivirus and will not be returned to the refrigerator after use, because the titer will drop sharply with each freezing/thawing cycle.
~For specific enrichment and detection parameters, please refer to Chapter 4 of this book;
2. Large-scale production of lentivirus
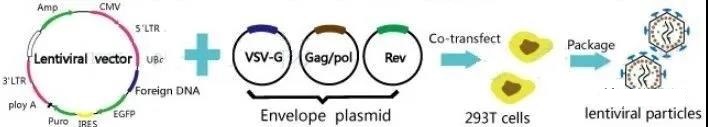
Lentivirus cell lines include adherent and suspension. The use of adherent production in large-scale production requires additional production units, and suspension culture is the more popular method. Here is an approach to adhere to the culture.
Adherent cell line, HEK293T/17 cell line is widely used as a lentivirus packaging system. Created by DuBridge et al. [15] By stably transfecting HEK293 cells, the HEK293T cell line contains a heat-sensitive version of the simian vacuolar virus 40 (SV40) large T antigen. Due to the presence of this T antigen, the transfection plasmid containing the SV40 origin of replication can be replicated, resulting in an increase in the lentiviral vector produced by HEK293T cells [12,16].
Suspension cell line, Jurkat is a T lymphocyte cell line used for virus titration in this protocol. This cell line was chosen because it represents a primary T lymphocyte infection in a more realistic way than the commonly used HEK293T/17 cells that are easier to transduce.
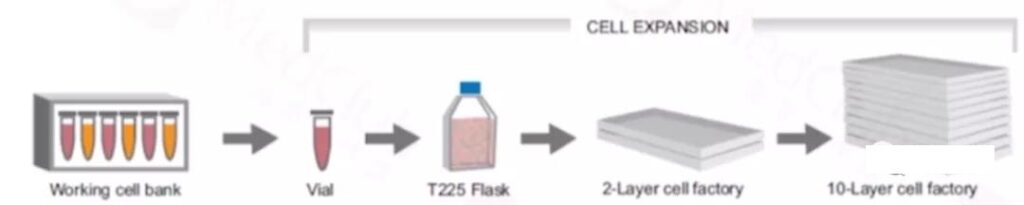
The cell line is resuscitated and passaged, then the plasmid is used for transfection and medium-changing culture, virus collection, filtration and concentration, aliquoting and storage (frozen virus is stable at 80°C for 6 months), while sampling is performed using flow cytometry Cytometer for testing.
~For specific production and testing parameters, please refer to Chapter 5 of this book;
3. Three methods of LV production: transient transfection, induced stable packaging cell line and stable production cell line
3.1 Transient transfection
1. Determine the cell density of the cell culture.
2. Centrifuge the cell culture (300g, 5 minutes) and gently resuspend it in fresh serum-free medium to target 1×106 cells/mL. Allow the cells to recover for about 5 minutes before proceeding with transfection.
3. Transfect cells with all four plasmids and transfection agent.
4. Target 1-1.5 μg of total plasmid DNA per 106 cells [8, 9]. The mass ratio of Tar packaging plasmid DNA, Rev plasmid DNA, envelope plasmid DNA and transgenic plasmid DNA is 1:1:1:2 [10]. The mass ratio of PEI and DNA was set to 2:1 [8].
5. After 6–24 h, centrifuge the cell culture, and then gently resuspend it in a fresh serum-free medium targeting the same working volume [6, 9].
6. Harvest cells 48–72 h after transfection [11]. Incubate the cells by centrifugation at 300-500g for 5-10 minutes, and filter the cell supernatant containing LV with a 0.45μm filter.
7. The filtered cell supernatant should be used immediately for further processing or analysis. Alternatively, it should be stored at 80°C.
3.2 Inducing stable packaging cell lines
1. Determine the cell density of the cell culture.
2. Centrifuge the cell culture (300 g, 5 minutes), and gently resuspend in fresh serum-free medium to target 1 × 106 cells/mL. Allow the cells to recover for about 5 minutes before proceeding with transfection.
3. Transfect cells with transgenic plasmid DNA and transfection agent.
4. Target 1–1.5μg plasmid DNA per 106 cells [8,9]. The mass ratio of PEI and DNA was set to 3:1 [6].
5. After 4 hours, induce with an appropriate concentration of inducer [6] (for example, 1μg/mL doxycycline and 10μg/mL subtilisate).
6. After 6-24 hours of transfection, the cells are cultured by centrifugation, and gently suspended in fresh serum-free medium, with an appropriate concentration of inducer in the medium for the same workload [6,9].
7. Harvest cells 48–72 h after transfection [11]. Incubate the cells by centrifugation at 300-500g for 5-10 minutes, and filter the cell supernatant containing LV with a 0.45μm filter.
8. The filtered cell supernatant should be used immediately (see Note 9) for further processing (see Note 10) or analysis (see Note 11). Alternatively, it should be stored at 80°C.
3.3 Induction of stable production cell lines
1. Determine the cell density of the cell culture.
2. Centrifuge the cell culture (300 g, 5 minutes), and gently resuspend in fresh serum-free medium to target 1×106 cells/mL. Allow the cells to recover for about 5 minutes before continuing the induction.
3. Use an appropriate concentration of inducer (for example, 1μg/mL doxycycline and 10μg/mL cumate) to induce cells.
4. Harvest cells 48–72 h after induction [11]. The cells were cultured by centrifugation at 300-500g for 5-10 minutes, and the cell supernatant containing LV was filtered using a 0.45μm filter.
5. The filtered cell supernatant should be used immediately (see Note 9) for further processing (see Note 10) or analysis (see Note 11). Alternatively, it should be stored at 80°C.
Some notes (these are summaries of experience, very precious):
1. A detailed list of commercially available serum-free media for HEK293 suspension culture: HyCell TransFx H (HyClone), SFM4TransFx-293 (HyClone), HEK medium (xell), FreeStyle 293 (Thermo Fischer).
2. For example, when using a 125 mL shake flask, the minimum culture volume of seeds is 15 mL, and the maximum culture volume is 20 mL. When using a 2000 mL shake flask, the minimum culture volume is 400 mL and the maximum culture volume is 600 mL.
3. The plasmid needs to be designed correctly. The ratio of plasmids may need to be adjusted because the properties of plasmids are variable and depend on many factors such as cell line, medium, transfection agent and other cultures.
4. Although it is necessary to perform downstream purification to remove host cell proteins, host cell DNA and other impurities to achieve high concentration and purity of LV, there is currently no standard method. The most commonly used concentration method is ultracentrifugation. At present, due to its scalability advantages, people have explored ultrafiltration and chromatography technologies, such as anion exchange and size exclusion [5].
5. After LV is generated, it needs to be quantified. Although the agreements between laboratories are different, there are several methods. For example, by titrating the p24 capsid protein concentration, the p24 enzyme-linked immunosorbent assay (ELISA) is used to measure total particles [12]. Recently, the droplet digital polymerase chain reaction (ddPCR) method has been developed to measure total particles by titrating vector genome copies. In order to determine the function or infection titer of LV with the reporter gene as the transgene, gene transfer assay (GTA) is usually used in combination with flow cytometry as a readout method. In clinical applications, PCR is usually used as a reading method to measure LV without reporter markers through GTA. In the past, qPCR has been widely used. However, due to its sensitivity and accuracy advantages, the trend is to turn to ddPCR [13].
6. A constitutive production cell line has been developed to avoid the use of inducers, thus eliminating the need to remove such supplements in downstream purification. Examples of constitutive cell lines are LentiPro 26 (a suspension system using a mutated, less active viral protease) [14] and WinPac (an adhesion system using a less cytotoxic envelope glycoprotein) [15].
(source:internet, reference only)
Disclaimer of medicaltrend.org



