Parkinson: Study on Gut microbiota-derived propionate
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Parkinson: Study on Gut microbiota-derived propionate
Parkinson: Study on Gut microbiota-derived propionate. Microbiome: Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease.
OCN can improve the motor dysfunction and the loss of dopaminergic neurons in Parkinson’s disease mice. Regulating the intestinal flora and increasing the level of propionic acid may be one of the mechanisms of OCN’s neuroprotective effect on Parkinson’s disease.
Guide
Parkinson’s disease (PD) is a neurodegenerative disease that cannot be completely cured. Osteocalcin (OCN) is a protein secreted by osteoblasts, which regulates brain function. The study found that ① in the 6-OHDA-induced PD mouse model, intraperitoneal injection of OCN can significantly improve motor dysfunction and dopaminergic neuron loss; ② OCN treatment can restore the imbalance of the intestinal flora of PD mice and make The abundance of Bacillus phylum increases and decreases the abundance of Firmicutes, increases the level of propionate-producing bacteria and fecal propionate; ③Antibiotic treatment significantly reduces the neuroprotective effect of OCN, and fecal bacteria transplantation experiments also show that the intestinal flora is mediated The neuroprotective effect of OCN; ④Oral propionate for 2 months can restore the motor function and dopaminergic neurons of PD mice in an enteric neuron-dependent manner; ⑤FFAR3 (propionate receptor) agonist is similar Neuroprotective effect.
Paper ID
- Paper name: Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease
- Journal: Microbiome
- IF: 11.607
- Publication time: 2021.1.31
- Corresponding author: Liu Jianmin
- Corresponding Author Unit: Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine
Experimental design
Parkinson’s disease (PD) is a neurodegenerative disease that cannot be completely cured. In recent years, the relationship between the gut microbiota and central nervous system diseases has attracted increasing attention. A number of clinical and animal studies have shown that the intestinal microflora of PD is different from that of healthy controls. Short-chain fatty acids (SCFAs) are the main metabolites of gut microbes, which transmit signals from the gut microbes to the host.
More and more data show that SCFAs have a positive effect on regulating brain function and the integrity of blood tissue barrier. However, studies have found that in the PD model, SCFAs are the main regulators of accelerating neuroinflammation and α-nucleinopathy. Therefore, it is necessary to look for microbiota or microbial derivatives to treat or improve PD. Osteocalcin (OCN) is a protein secreted by osteoblasts, which regulates brain function. OCN can penetrate the blood-brain barrier.
After 2 months of peripheral injection of OCN in old mice, OCN directly binds to neurons in the CA3 region of the hippocampus and completely restores the cognitive function of the mice. In the process of bone remodeling, the intestinal microflora has a regulatory effect on the activity of osteoblasts and osteoclasts. A recent finding showed that the serum level of OCN is related to the Chao index of the intestinal flora of patients with Crohn’s disease, which further indicates that OCN may affect the composition of the flora. Therefore, it is hypothesized that OCN can prevent sports injury and dopaminergic neuron loss by regulating the intestinal microflora of PD mice.
Results:
1 OCN can prevent 6-hydroxydopamine (6-OHDA)-induced motor injury and dopaminergic neuron loss in Parkinson’s disease mice
We first observed the effect of intraperitoneal injection of OCN in the 6-hydroxydopamine-induced PD mouse model. Behavioral tests showed that the movement distance and feeding frequency of Parkinson’s disease mice in the open field test were significantly reduced, and the left limb contact frequency was defective in the cylinder test.
Rotation test Compared with the control group, 6-OHDA incubation period of induced PD mice is reduced (Figure 1b). In the open field test and cylinder test of PD mice, administration of 4μg/kg OCN for 8 weeks significantly improved the dyskinesia of the experimental group but did not affect the control group (Figure 1b).
At the same time, experiments examined whether OCN also protects dopaminergic neurons in the substantia nigra (SN) and striatum. The immunostaining of tyrosine hydroxylase (TH) in the brains of PD mice on the side of 6-OHDA injection for identifying substantia nigra dopaminergic neurons was reduced by nearly 70% compared with the control group, while OCN treatment could significantly prevent the dopaminergic activity of PD mice Decrease of neurons (Figure 1c).
Consistent with this, immunostaining and Western blotting of the striatum also showed that the TH-positive fibers and TH protein levels on the injection side of the striatum were significantly lower than those of the control group mice that recovered after OCN treatment (Figure 1d). However, administration of OCN (40μg/kg) did not significantly increase the number of dopaminergic neurons in the SN and striatum of PD mice (Figure 1c, d). After 4μg/kg OCN intervention, the dopaminergic neurons in the SN and striatum of the control group mice did not change. Therefore, this evidence shows that administration of 4 μg/kg OCN can prevent 6-OHDA-induced dyskinesia and dopaminergic neuron loss in PD mice.
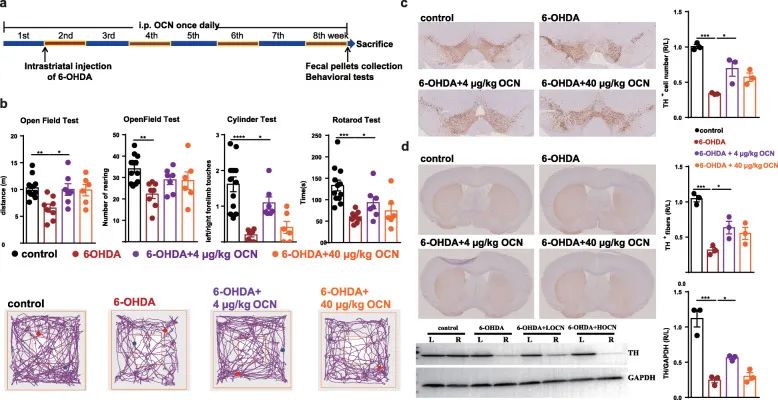
Figure 1 OCN administration can prevent 6-OHDA-induced motor injury and dopaminergic neuron loss in Parkinson’s disease mice.
(A) Experimental design of OCN intervention in 6-OHDA-induced PD mice.
b) Open field test, cylinder test and rotation test.
(C) SN immunostaining TH-positive neurons.
(D) Striatum immunostaining and western blotting TH-positive fibers and TH protein levels.
2 Intestinal flora mediates the neuroprotective effect of OCN on PD
To test whether the neuroprotective effect of OCN on PD depends on the intestinal flora, we eliminated the intestinal flora by using antibiotics 4 weeks before taking OCN (Figure 2a). The reduction of intestinal microbes blocked the improvement in motor function induced by OCN, including the distance of the mice in the open field test, the frequency of rearing, the frequency of contact with the left limb in the cylinder test, and the incubation period in the rotation test (Figure 2b).
In addition, we examined whether the loss of intestinal microbes affects the effects of 6-OHDA and OCN on the loss of dopaminergic neurons in PD mice. The immunostaining of TH in SN showed that the damage of dopaminergic neurons induced by 6-OHDA was not affected by the decrease of intestinal microbes.
However, in 6-OHDA-induced PD mice, the loss of gut microbes prevented OCN from preventing the loss of dopaminergic neurons (Figure 2c). The results of immunostaining and western blotting in the striatum confirmed that the loss of intestinal flora can block OCN-induced protection against dopaminergic neuron loss (Figure 2d).
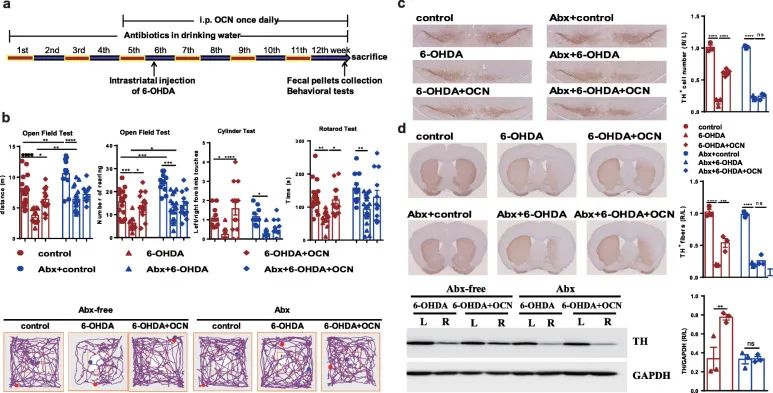
Figure 2 Antibiotic pretreatment (ABX) blocked the improvement of OCN-induced dyskinesia and dopaminergic neuron loss in 6-OHDA Parkinson’s disease mice.
(A) The experimental design of 6-OHDA-induced PD mice with antibiotic treatment and 4 μg/kg OCN administration.
(B)Open field test, cylinder test and rotation test.
(C) SN immunostaining TH-positive neurons.
(D) Striatum immunostaining and western blotting TH-positive fibers and TH protein levels.
In order to further support the view that changes in the intestinal flora mediate the neuroprotective effect of OCN on PD, the fecal flora of the control group, 6-OHDA group and 6-OHDA+ OCN group were transplanted to a small group that was pretreated with antibiotics for 5 weeks. In the mouse. Two weeks after fecal transplantation, we compared the motor function of different groups of mice (Figure 3a). Compared with FMT6-OHDA+ OCN, the FMT6-OHDA group is
Pole test
The total decay time of is extended, while the delay in the round-robin test is significantly reduced (Figure 3b). In the open field test, the movement distance and feeding frequency of mice did not change (Figure 3b). Compared with 6-OHDA, mice receiving 6-OHDA+OCN microbial flora improved their locomotor ability, indicating that intestinal flora may mediate OCN to improve motor dysfunction in PD mice (Figure 3b). Therefore, antibiotic treatment and fecal microbiota transplantation experiments have shown that the intestinal microbiota is necessary for the protective effect of OCN on PD.
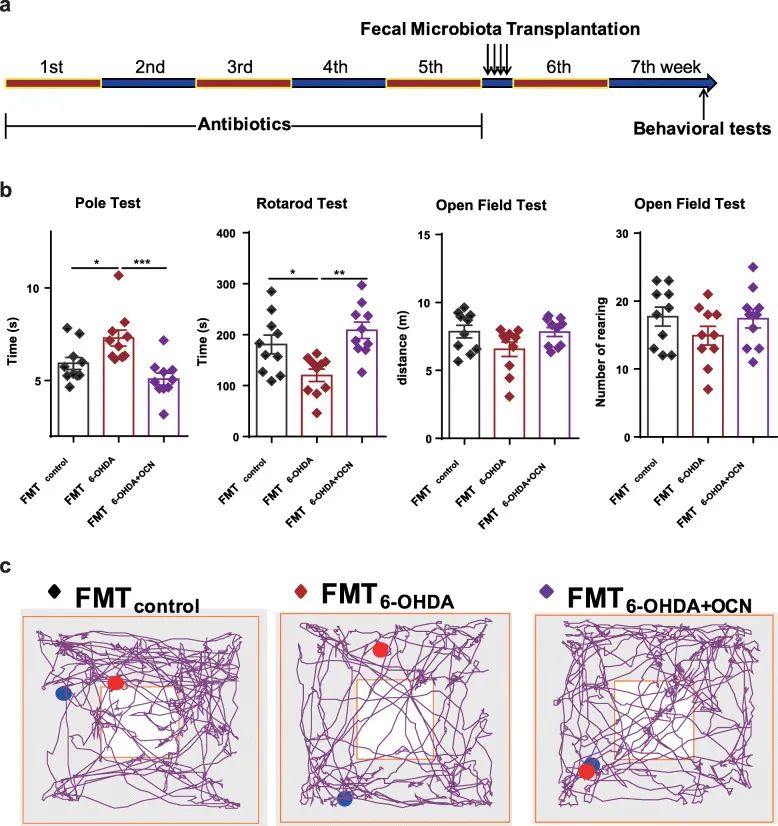
Figure 3 In mice with deficient gut microflora, the PD microflora induces PD-like dyskinesia. (A) Experimental design of fecal microbial transplantation. (B) Open field test, pole test and cycle test. (C) There are representative traces in the open field test.
3 The changes of intestinal microflora in 6-OHDA-induced Parkinson’s disease mice and OCN-treated Parkinson’s disease mice
Through 16SrRNA sequencing, the composition of the intestinal microflora was evaluated. The Simpson and Shannon indexes were not significantly different between the groups, indicating that 6-OHDA or 4μg/kgOCN treatment had no effect on the diversity of mouse intestinal microflora, while UniFrac principal coordinate analysis showed that 6-OHDA-induced PD mouse intestines The composition of the tract microbes was significantly different from that of the control group and the control group (P<0.05), while the 6-OHDA and 4μg/kgOCN treatment groups had no significant difference in the diversity of the intestinal microflora of mice (P>0.05).
UniFrac principal coordinate analysis showed that the intestinal microbial composition of PD mice induced by 6-OHDA was significantly different from that of the control group (P<0.05), while OCN treatment restored the microbial community structure of Parkinson’s disease mice to the level of the control group (Figure 4a). In the heat map and Venn diagram (Figure 4b, c), we found that the 6 taxa of the 6-OHDA group had significant changes compared with the control group.
Compared with the control group, the relative abundances of Bacteroidetes, S24-7, Rikenellaceae, and Erysipelotrichaceae in Parkinson’s disease mice induced by 6-hydroxydopamine were significantly reduced, while the relative abundances of Firmicutes, Lachnospiraceae, and unclassified Clostridiales were significantly increased. However, OCN administration significantly reversed this change in Parkinson’s disease mice (Figure 4d).
Through PICRUST function analysis, it was found that the intestinal microbiome of PD mice induced by 6-OHDA significantly reduced the ability to produce propionic acid compared with the control group, which can be used as a proof of the decrease in K01847 RA (Figure 5a). In addition, the RAs of KO related to butyrate production also changed. The content of K00634 was significantly reduced, and the content of K00074 was significantly increased; while the content of K01034 and K01035 did not change significantly (Figure 5a).
The RAs of the main KOs related to acetic acid production did not differ between the control group and the 6-OHDA group (Figure 5a). It is worth noting that OCN administration successfully reversed these changes in Parkinson’s disease mice. However, the KOs of control mice were not affected by OCN administration. Therefore, the intervention of OCN can change the microflora of PD mice and may increase the potential of bacteria to produce propionic acid.
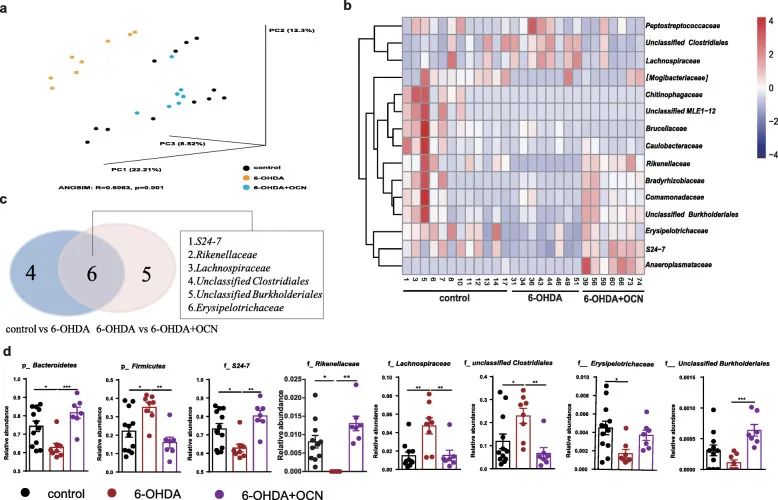
Figure 4 The regulation effect of OCN administration on 6-OHDA-induced intestinal microflora disorders in PD mice. (A) A principal coordinate analysis (PCoA) diagram based on unweighted UniFrac.
b) Heat map. (C) Venn diagram. (D) The relative abundance of Bacteroidetes and Firmicutes at the phylum level and the family level.
4 The effect of OCN on the level of propionic acid in the stool of mice with Parkinson’s disease induced by 6-OHDA
In order to further study whether bacterial SCFAs have changed, we used gas chromatography/mass spectrometry (GC/MS) to analyze the content of SCFAs in feces. The results showed that the fecal propionic acid content of 6-OHDA-induced Parkinson’s disease mice was significantly lower than that of the control group, and OCN treatment significantly reversed this change (Figure 5b). There were no significant differences in the levels of acetate and butyrate in the feces of the three groups (Figure 5b).
OCN administration did not affect the levels of these three SCFAs in the feces of control mice. In addition, correlation analysis showed that propionic acid levels in feces were positively correlated with S24-7 and Rikenellaceae, but negatively correlated with Lachnosipraceae and unclassified Clostridiales (Figure 5c), while the other two SCFAs were associated with Pd-deficient groups S24-7 (r= 0.4961, P=0.0010) and Rikenellaceae (r=0.5063, P=0.0098) RAs were positively correlated, suggesting that the changes in these microorganisms may be related to changes in fecal propionic acid levels. More importantly, the level of fecal propionic acid was positively correlated with the motor function parameters of the open field test, cylinder test, and rotating rod test (Figure 5d). Therefore, the intestinal microbe-mediated neuroprotection of OCN may be related to its potential to regulate the production of propionic acid in the intestinal microflora of PD mice induced by 6-OHDA.
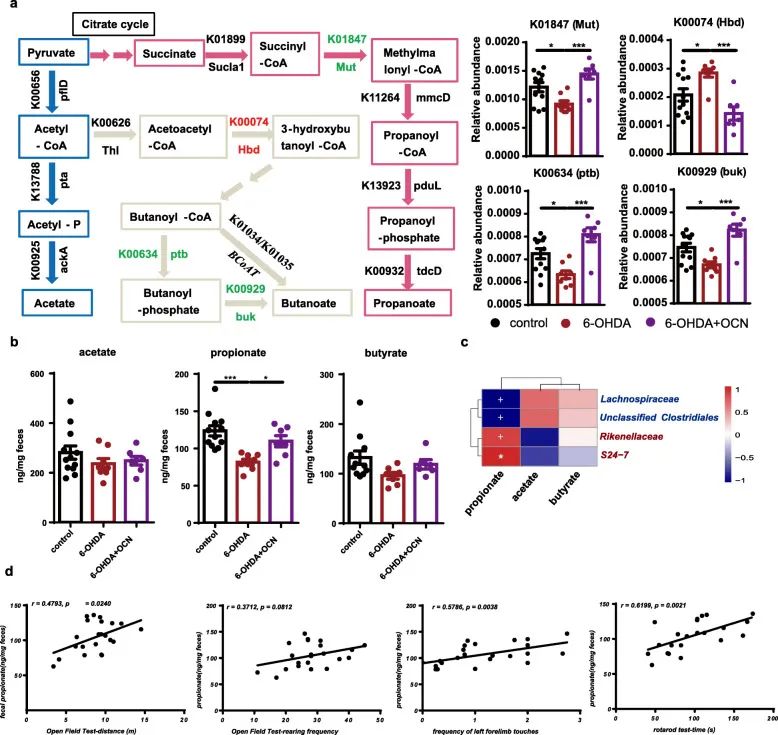
Figure 5 OCN administration can increase fecal propionic acid levels in PD mice induced by 6-OHDA. (A) Reveal the metabolic pathways of acetate, propionate and butyrate synthesis, the corresponding KOs and genes, as well as the bar graphs of the relative abundances of K01847, K00634, K00929, and K00074. (B) Bar graph of the levels of acetic acid, propionic acid and butyric acid in feces of each group. (C) Heat map. (D) Correlation analysis chart of propionic acid excretion and motor function.
5 Propionic acid and FFAR3 agonists prevent 6-hydroxydopamine-induced motor injury and dopaminergic neuron loss in Parkinson’s disease mice
In order to further verify the relationship between propionic acid and the improvement of motor function in Parkinson’s disease mice, we added sodium propionate to the drinking water of 6-OHDA-induced Parkinson’s disease mice. Compared with the control group, propionic acid intervention caused a significant increase in fecal propionic acid levels in 6-OHDA-induced Parkinson’s disease mice, but no significant changes in serum propionic acid levels (Figure 6a).
Importantly, oral propionic acid effectively improved the motor function of mice in the open field test and cylinder test, but the rotation test did not improve (Figure 6b). At the same time, oral propionic acid can prevent the loss of nearly 40% of dopaminergic neurons in the brain of Parkinson’s disease mice injected with 6-hydroxydopamine (Figure 6c). Propionic acid-induced TH protein levels in the striatum also showed a similar pattern of changes (Figure 6d). Therefore, we speculate that propionic acid may be a signal derived from the intestinal microflora, contribute to the development of Parkinson’s disease, and may be a target of OCN.
FFAR3 is the main receptor type that mediates the protective effects of propionic acid. 6-OHDA-induced Parkinson’s disease mice were given FFAR3 agonist (AR420626, 0.1 mg/kg) once a day for 8 weeks (Figure 7a). Like the effect of propionic acid on Parkinson’s disease mice, the intervention of AR420626 effectively increased the breeding of 6-OHDA-induced Parkinson’s disease mice in the open field test.
The number of times, but did not significantly increase the movement distance; significantly improved the motor function in the pole test and the cycle test, but did not improve the motor function in the cylinder test (Figure 7b). At the same time, intragastric administration of AR420626 can prevent the loss of 20% of dopaminergic neurons on the 6-OHDA side injected into the brain of Parkinson’s disease mice (Figure 7c), and restore the level of striatal TH protein (Figure 7d). Therefore, AR420626, an agonist of FFAR3, mimics the neuroprotective effects of propionic acid. We speculate that propionic acid modified by intestinal microbes may partially act as FFAR3 agonists, transmitting the protective signal of OCN to the nervous system to prevent the occurrence of PD.
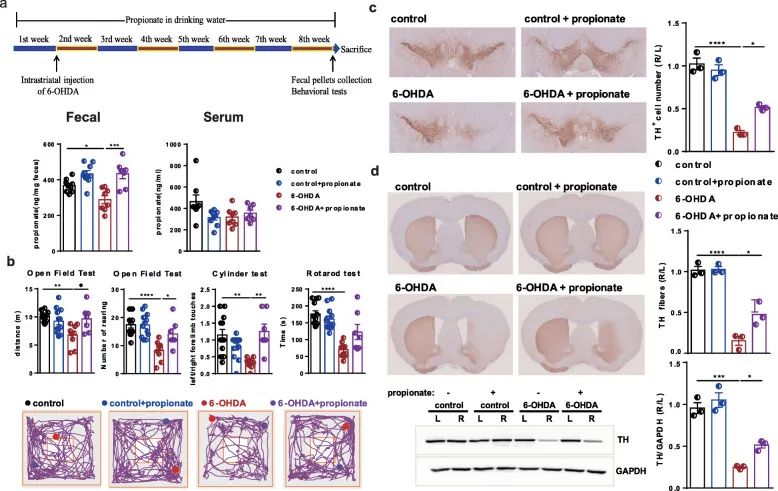
Figure 6 Oral propionic acid prevents dyskinesia and dopaminergic neuron loss in 6-OHDA-induced PD mice. (A) Experimental design of propionate intervention in PD mice. (B)
Open field test, cylinder test and rotation test. (C) SN immunostaining TH-positive neurons. (D) Striatum immunostaining and western blotting TH-positive fibers and TH protein levels.
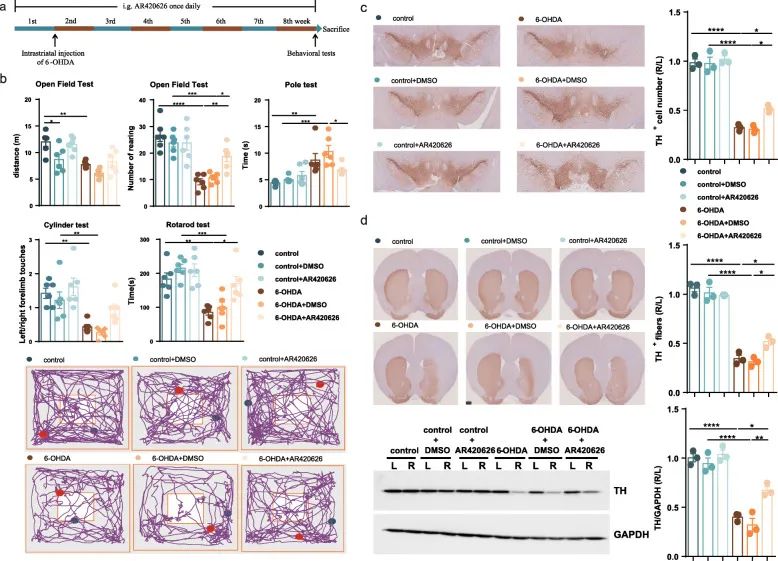
Figure 7 In 6-OHDA-induced PD mice, administration of FFAR3 agonist prevents movement disorders and dopaminergic neuron loss. (A) Experimental design of FFAR3 agonist intervention in PD mice. (B) Open field test, cylinder test and cycle test. (C) SN immunostaining TH-positive neurons. (D) Striatum immunostaining and western blotting TH-positive fibers and TH protein levels.
6 The enteric nervous system may mediate the neuroprotective effect of propionic acid on 6-OHDA-induced PD mice
Since FFAR3 activation improved the motor deficits in PD mice, we measured the expression of FFAR3 in different tissues. We found that the relative expression of FFAR3 in the jejunum, ileum, and colon was much higher than that in the cortex, hippocampus, and cerebral striatum (Figure 8a).
Since propionic acid exerts its effects by stimulating enteroendocrine L cells to release GLP-1 or activating the enteric nervous system (ENS) to transmit its signals to the brain, we first measured serum based on the neuroprotective effect of GLP-1 on PD The level of GLP-1. However, no significant differences were observed between Parkinson’s disease mice and control mice, or between propionic acid-treated Parkinson’s disease mice and untreated Parkinson’s disease mice (Figure 8b) .
Then, in order to test whether propionic acid can act on ENS, we gavage 6-hydroxydopamine-induced Parkinson’s disease mice with 3 mg/kg cisplatin (a known enteric neurotoxin), and make them take C PGP9.5-positive intestinal cells are depleted before acid. In these mice with depleted intestinal cells, we found that the protective effect of propionic acid on dopaminergic neuron loss was no longer significant (Figure 8c, d). Therefore, we speculate that propionic acid can be used as a FFAR3 agonist for ENS, and exert neuroprotective effects on 6-OHDA-induced Parkinson’s disease mice.
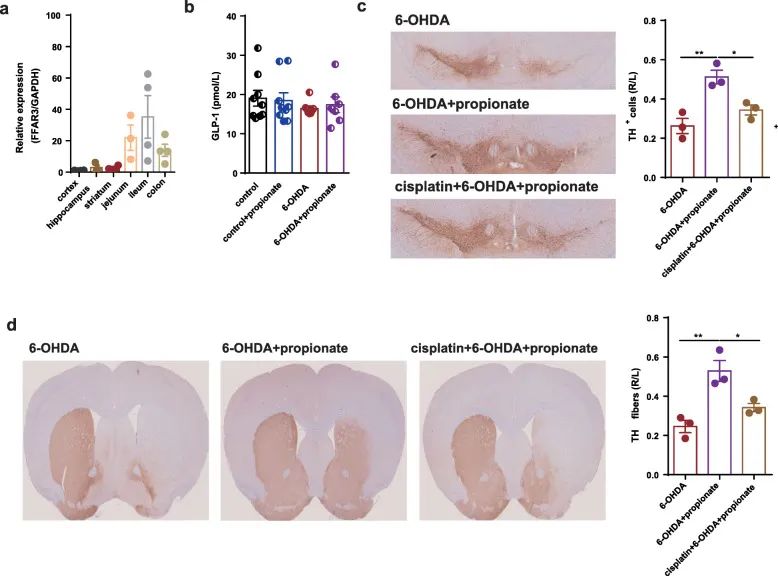
Figure 8 The enteric nervous system mediates the neuroprotective effect of propionic acid on 6-OHDA-induced PD mice. (A) Relative expression of FFAR3 in cortex, hippocampus, striatum, jejunum, ileum and colon. (B) Bar graph of serum Glp-1 levels between different groups. (C) SN immunostaining TH-positive neurons. (D) Striatum immunostaining and western blotting TH-positive fibers and TH protein levels.
Discussion:
Research results show that OCN can activate FFAR3 in enteric neurons by promoting the production of microbial propionic acid, thereby exerting its protective effect on Parkinson’s disease. Using the 6-OHDA-induced mouse model of Parkinson’s disease, it is confirmed that OCN can prevent the motor injury and dopaminergic neuron loss in Parkinson’s disease mice. The data further show that OCN can play a protective role by regulating the microbial metabolism of SCFAs and increasing the level of propionic acid in the intestinal microflora of PD mice. In addition, oral propionic acid has a protective effect on Parkinson’s disease, suggesting that propionic acid is a potential target for Parkinson’s disease intervention. Data confirms that propionic acid mainly acts on FFAR3 in enteric neurons and exerts its neuroprotective effect on Parkinson’s disease.
A number of clinical and animal studies have investigated the correlation between intestinal microbiota disorders and Parkinson’s disease, and the results have been inconsistent with the observations. The changes in the gut microbiota found in 6-OHDA-induced PD mice in our study are similar to those observed in rotenone-induced PD mouse models, but different interventions and administration routes result in changes in the gut microbiota. The changes are different. OCN can reverse the above-mentioned microbial changes in PD mice in this study. In addition, since the effect of OCN in improving dyskinesia and dopaminergic neuron loss in Parkinson’s disease mice is weakened in mice without intestinal microflora, and the phenotype of Parkinson’s disease can be transferred by fecal transplantation, it is believed that OCN is in The 6-OHDA-induced Parkinson’s disease mouse model relies on the intestinal microflora to play a neuroprotective effect.
The microbe-gut-brain axis has recently attracted great research attention, and SCFAs may be the key signal transmitter. A recent clinical study showed that SCFAs in stool samples of Parkinson’s disease patients were significantly reduced compared with healthy controls. Oral SCFAs in sterile Parkinson’s disease mice promoted neuroinflammation and motor dysfunction. However, although the altered KOs of PD mice produced butyric acid and propionic acid at the same time, and were restored by OCN, only propionic acid in the feces changed significantly, indicating that in the study, propionic acid may dominate the intestinal effects of OCN on PD Microflora related effects. In addition, studies on oral propionate supplementation rescued the motor injury and dopaminergic neuron loss in Parkinson’s disease mice, further suggesting that increasing the production of microbial propionic acid may mediate the effects of OCN in the intestinal microflora of Parkinson’s disease. Play a leading role.
According to reports, propionic acid is mainly produced by Bacteroidetes. Our results found that Bacteroidetes, especially S247 and Rikenellaceae, were significantly positively correlated with fecal propionic acid levels. In addition, a variant KO was found in the succinate pathway for propionic acid production, which was positively correlated with the RAs of Bacteroidetes, S24-7 and Rikenellaceae, indicating that these gut microbes may carry a mutase encoding methylmalonyl-CoA Genes to regulate the succinate pathway.
We believe that the dosage and administration of propionic acid may be important factors in determining the efficacy of propionic acid. In this study, we administered 200 mM sodium propionate orally to mice with reduced fecal propionic acid levels. After the intervention, the fecal propionic acid level of 6-OHDA-induced Parkinson’s disease mice rose to the level of the control group.
According to reports, propionic acid mainly acts through one of two free fatty acid receptors, FFAR2 or FFAR3, and is widely expressed in multiple organs, including enteroendocrine L cells and the peripheral nervous system. Therefore, propionic acid can act by stimulating the release of GLP-1 from enteroendocrine L cells or activating the peripheral nervous system to transmit its signals to the brain. According to reports, GLP-1 has clinical benefits in the treatment of moderate PD, but in our study, there was no significant difference in serum GLP-1 levels between groups, so the possibility of GLP-1 related effects was ruled out. Next, in order to further prove the importance of ENS in the regulation of the effect of propionic acid on Parkinson’s disease, we used enteric neurotoxin to ablate enteric neurons. After ablation of intestinal neurons, the neuroprotective effect of propionic acid was weakened, suggesting that ENS may mediate the protective effect of propionic acid on serotonin-induced Parkinson’s disease mice. FFAR3 is the only propionic acid receptor in ENS. It is activated by propionic acid and induces IGN through enterocerebral neural circuits. FFAR3 agonists can mimic the neuroprotective effect of propionic acid on Parkinson’s disease mice. Therefore, FFAR3 may be a sensor of propionic acid in ENS, and propionic acid may exert its neuroprotective effect by activating FFAR3 in ENS.
Comments:
OCN can improve the motor dysfunction and the loss of dopaminergic neurons in Parkinson’s disease mice. Regulating the intestinal flora and increasing the level of propionic acid may be one of the mechanisms of OCN’s neuroprotective effect on Parkinson’s disease.
Neuroendocrine FFAR3 may Mediates the neuroprotective effect of propionic acid on Parkinson’s disease. Of course, there are some limitations in the study. The study mainly shows that OCN protects mice with Parkinson’s disease rather than treats them.
At the same time, in the PD model induced by MPTP and the PD model induced by rotenone, the protective effects of OCN and propionic acid on PD were not detected. Therefore, people need more in-depth research and clinical investigation to test the mechanism of OCN on PD.
(source:internet, reference only)
Disclaimer of medicaltrend.org



