The new dual immunotherapy is expected to treat head and neck cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The new dual immunotherapy is expected to treat head and neck cancer
The new dual immunotherapy is expected to treat head and neck cancer. The new dual immunotherapy is expected to be the first-line treatment for head and neck cancer, and it can also reduce drug resistance!

Squamous cell carcinoma of the head and neck is the sixth most common cancer in the world. It includes cancers of the oral cavity, nasal cavity, pharynx and larynx.
Recently, the FDA awarded the soluble LAG-3 protein Eftilagimod alpha (also known as IMP321) fast track designation for the first-line treatment of patients with recurrent/metastatic head and neck squamous cell carcinoma (HNSCC).
Eftilagimod alpha is a soluble LAG-3 fusion protein that can be combined with PD-1/PD-L1 inhibitors to improve the efficacy of immunotherapy resistant patients.
▌The sixth largest cancer in the world: head and neck cancer
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world. In 2018, there were approximately 800,000 newly diagnosed patients and approximately 450,000 patients died as a result.
Head and neck cancer is a “large family”, including cancers of the mouth, nose, pharynx, and larynx. Among them, more than 90% are HNSCC.
The occurrence of head and neck cancer has a lot to do with bad lifestyle habits, such as smoking, alcoholism or betel nut chewing. In addition, the incidence of oropharyngeal cancer associated with human papillomavirus (HPV) infection is also increasing year by year. The main risk factor is oral sex, which needs attention.
In June last year, the FDA approved a new indication for the nine-valent HPV vaccine to prevent oropharyngeal cancer and other head and neck cancers caused by HPV, which made it possible to prevent some head and neck cancers.
For patients diagnosed with head and neck cancer, in addition to surgery and chemotherapy, there are also recent hot immunotherapies such as nivolumab and pembrolizumab.
However, PD-1/PD-L1 inhibitors are only effective for some patients, and among the responding patients, 10%-15% of them still develop resistance, and the combination therapy is expected to improve the efficacy of immunosuppressants and reduce resistance. The occurrence of medicine.
▌LAG-3 fusion protein: Eftilagimod Alpha
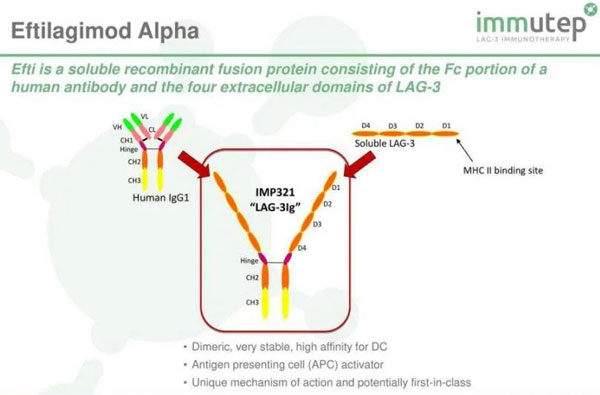
Eftilagimod alpha is a soluble LAG-3 fusion protein that has been shown to improve the remission rate of pembrolizumab in a clinical trial in combination with “K drug” pembrolizumab in the treatment of non-small cell lung cancer.
LAG3 (lymphocyte activation gene 3) is an immune checkpoint receptor protein that can regulate the signal pathway of T lymphocytes and antigen-presenting cells (APCs). Preclinical studies have shown that blocking LAG-3 can promote T cell proliferation and enhance its immune function.
▌The objective remission rate is 39%, and the combination medication has better effect!
This identification is based on the positive results of the TACTI-002 Phase 3 trial. The trial enrolled 88 patients, including patients with non-small cell lung cancer. Among them, there are 28 patients in the HNSCC cohort, and the efficacy and safety of 18 patients can be assessed.
With a median follow-up time of 9.5 months, the objective response rate (ORR) of patients in the Eftilagimod alpha combined with pembrolizumab treatment group was 38.9%. Among them, the disease control rate was 50%, and about 11% of patients had complete remission after treatment.
The median PFS of the HNSCC cohort was 4.26 months, and the 9-month and 12-month OS rates were 67% and 60%, respectively. At the time of the data cutoff, 7 patients with HNSCC were still receiving treatment.
The results show that even in patients with low PD-L1 expression and usually not responding to treatment, Eftilagimod alpha has a profound and long-lasting response to patients with head and neck squamous cell carcinoma.
In addition, the combination therapy is well tolerated. Based on the results of this trial, a phase 3 trial explores the efficacy and safety of Eftilagimod alpha combined with pembrolizumab as a first-line treatment for HNSCC.
▌Related drug news: new dosage regimen approved
In addition, recently, the FDA also approved a new dosage regimen of targeted anticancer drug cetuximab: intravenous infusion of 500 mg/m2 for 2 hours, once every 2 weeks, for the treatment of wild-type K-Ras and positive EGFR expression. Of metastatic colorectal cancer (mCRC) and squamous cell carcinoma of the head and neck (SCCHN).
Cetuximab is the world’s first monoclonal antibody targeting EGFR, and it was awarded the first batch in the United States in 2004.
So far, the drug has been approved for marketing in more than 100 countries around the world, and the approved indications include patients with advanced or metastatic head and neck cancer, wild-type K-Ras, and metastatic colorectal cancer with positive EGFR expression.
The approval of the new dose of cetuximab provides clinicians and patients with a new treatment plan.
(source:internet, reference only)
Disclaimer of medicaltrend.org



