Tumor CD8+ T cell dysfunction-driving factors….
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Tumor CD8+ T cell dysfunction-driving factors, regulation, markers, repair
Tumor CD8+ T cell dysfunction-driving factors, regulation, markers, repair. The clinical use of CAR-T and Immune Checkpoint Blocking (ICB) therapies requires a more accurate understanding of the functional status of T cells infiltrated in tumors, such as whether CD8+ T cells are functioning normally or are in early dysfunction ( early dysfunctional state), or it is already late dysfunctional state. CD8+T cell dysfunction is a hindering factor for immune cell therapy and immune checkpoint therapy.
Transcription factors regulate T cell dysfunction
CD8+ T cell dysfunction can be divided into predysfunctional state, early dysfunctional state, and late dysfunctional state on the time axis.
CD8+ T cell dysfunction is tightly regulated by various transcription factors, including EOMES (eomesodermin homologue), T-BET (T-box expressed in T cells; coded by TBX21), BLIMP1 (B lymphocyte- induced maturation protein 1. PRDM1 Encoding), MAF, TOX, TCF1 (cell-specific transcription factor 1, also known as TCF7; encoded by TCF7).
In mouse studies, the expression levels of these transcription factors are interdependent. For example, deletion of EOMES leads to an up-regulation of TCF1 levels and a down-regulation of TOX. Similarly, TOX expression is positively correlated with Prdm1 expression, while MAF is negatively correlated with Prdm1. This shows that the dysfunction is controlled by a complex regulatory network.

Intratumoral CD8+ T cell dysfunction and its markers

CD8+ T cell status and markers in tumor
The naive-like cells described in non-small cell lung cancer (NSCLC), liver cancer, hepatocellular carcinoma, colorectal cancer, basal cell carcinoma and melanoma have strong similarities with central memory-like cells (such as CCR7, TCF7 high expression , Weak cytotoxicity).
Low cytotoxicity, expression of inhibitory molecules, and relatively low clonality are the characteristics of the predysfunctional state of CD8+T such as NSCLC, liver cancer, and melanoma.
The tissue-resident memory T cells (TRM cells) described in triple-negative breast cancer and HAVCR2TRM cells in NSCLC are consistent with the dysfunctional state described in all other studies.
In some tumors, pre-cell dysfunction and cytotoxicity coexist.
The pre-dysfunctional cell state is mainly driven by tumor-specific factors, such as tumor antigen recognition and/or tumor-specific environmental factors (differentiation driven by tumor microenvironmental factors).
The cytotoxic state differentiation process is not strictly tumor-specific (differentiation independent of the tumor microenvironment).
Cytotoxic effector T cells and pre-dysfunctional T cells may come from different populations, but further studies are needed to clarify whether cytotoxic effector cells are indeed derived from different cell pools, or whether they are dysfunctional in some cases. The shafts are connected (as shown by the dashed double-headed arrow). Trajectory and T cell receptor (TCR) sharing analysis shows that pre-dysfunctional and dysfunctional cells form a continuum of cell states, rather than distinguishing two populations well.
Drivers of CD8+ T cell dysfunction

- Suboptimal priming
- Continuous TCR stimulation drive
- Inhibitory environment (eg IL-10, TGF-β, hypoxia)
T cell initiation is described as occurring in the lymph nodes, but it may also occur in the tumor site (tertiary lymphatic structure (TLS)).
PD-1 antibody repairs CD8+ T cell dysfunction

PD-1 treatment
- Enhance pre-dysfunction and early dysfunction cell proliferation
- Reverse and enhance pre-dysfunction and early dysfunction cell effector function
- Enhance the effector function of advanced dysfunction cells
- Generating new cell states through clonal replacement
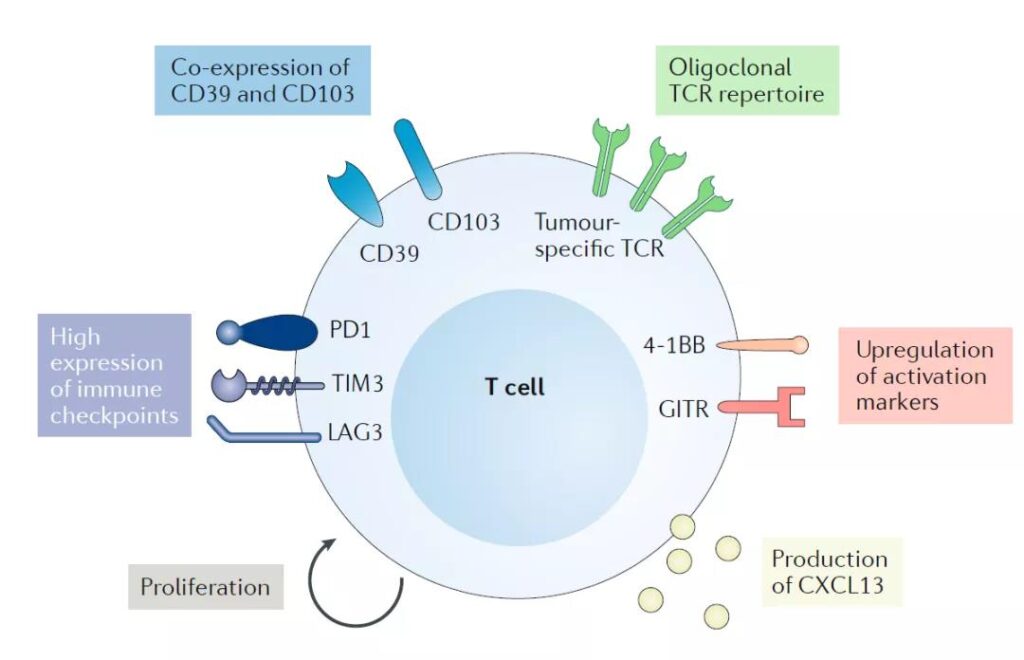
Characteristics of tumor-reactive T cells
- Activate marker up-regulation, such as 4-1BB, GITR, etc.
- Highly express immune checkpoints and enhance the response to PD-1/PD-L1 antibodies
- Increased proliferation
- Produce CXCL13, chemotactic B cells, help to form tertiary lymphoid tissue in the tumor area
Expert Comments:
CD8+ T cell dysfunction in tumors is driven by factors such as non-optimal activation, continuous stimulation of tumor antigens, and inhibitory microenvironment. Treatments such as PD-1 can reverse the insufficiency of CD8+ T cells and restore functional homeostasis.
(source:internet, reference only)
Disclaimer of medicaltrend.org



