The analysis of COVID-19 mRNA patent is more exciting than imagined
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The analysis of COVID-19 mRNA patent is more exciting than imagined
The analysis of COVID-19 mRNA patent is more exciting than imagined. The key to the protection of intellectual property rights for the construction of two mRNA vaccines is to encode mRNA, construct the new coronavirus spike protein and related patents for the delivery of mRNA in vivo.
COVID-19 has ravaged the world for nearly a year and a half. As of May 21, there are approximately 15.85 million confirmed patients worldwide, with a total of approximately 166 million confirmed cases and approximately 3.44 million deaths. Although the epidemic situation in some countries is basically under control, there are still ups and downs. As of May 16, some countries has 2372 confirmed patients (including 1991 from Taiwan, 73 from Hong Kong, and 1 from Macau), with a total of 205,962 confirmed cases and 4,861 deaths.
Vaccines with higher protection effectiveness and safer are scarce in the world and developed by various pharmaceutical companies. From the published clinical data, the mRNA vaccine is the safest and most effective COVID-19 vaccine today. There is no one. The protective effect of one dose of the mRNA vaccine BNT162b2 is as high as 95%.
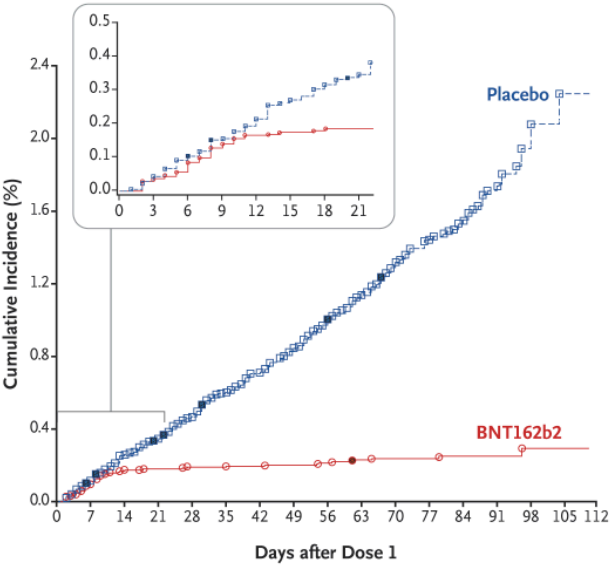
However, as the awareness of intellectual property rights has been deeply rooted in the hearts of the people, any innovative product needs the escort of intellectual property rights, especially pharmaceuticals. Historically, no company can avoid a cliff-like decline in the sales of blockbuster drugs when patents expire. For the mRNA in the fire today, patents are also the key guardians of the development of medicines (including vaccines).
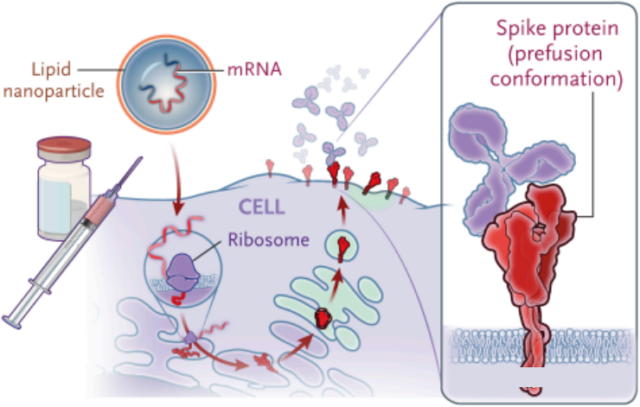
Take the two new coronavirus mRNA vaccines on the market as an example, Pfizer/Biontech’s BNT162b2 (tozinameran) and Moderna’s mRNA-1273. Both mRNA vaccines are mRNA encoding the new coronavirus spike protein. After the vaccine is injected into the human body, it is produced in the human body. Coronavirus spike protein, which in turn activates the human immune system.
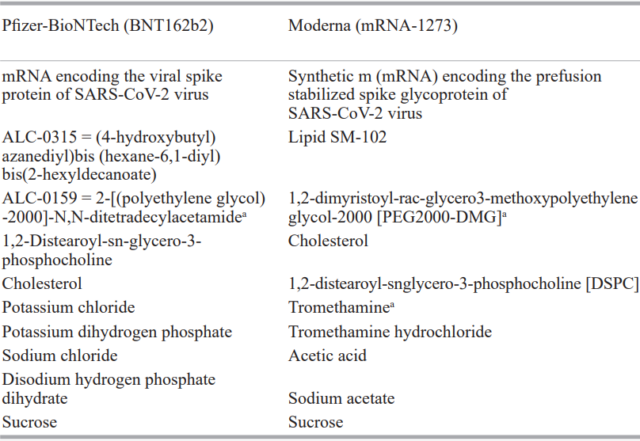
The composition of BNT162b2 and mRNA-1273 is shown in the following table.
The key to the protection of intellectual property rights for the construction of two mRNA vaccines is to encode mRNA, construct the new coronavirus spike protein and related patents for the delivery of mRNA in vivo.

On May 13, Mario Gaviria of the University of Michigan at Ann Arbor and Burcu Kilic, a citizen of Washington, jointly published an article in Nature Biotechnology, a detailed summary of COVID-19 mRNA vaccine technology field and related patent attribution.
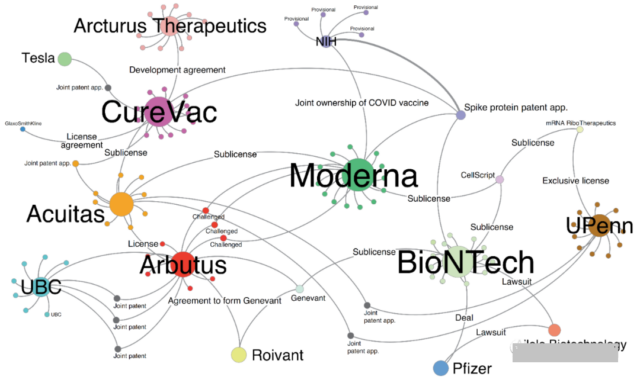
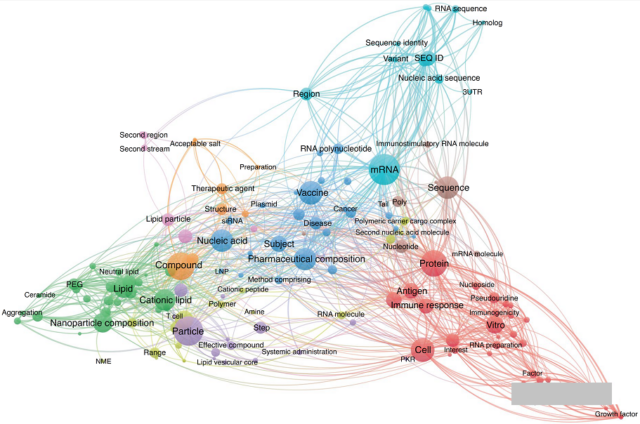
As the founders of mRNA technology, Professor Katalin Karikó and Drew Weissman of the University of Pennsylvania (UPenn) have core patents for encoding mRNA. In 2017, UPenn transferred the patent for encoding mRNA to mRNA RiboTherapeutics and its subsidiary CellScript, which continued to transfer the patent license. To Moderna and BioNTech.
Before the outbreak of COVID-19, in view of the fact that each outbreak of coronaviruses such as SARS and MERS will cause unpredictable casualties, in 2019, the National Institutes of Health (NIH), led by the famous virus expert Fauci, relied on strong research and development capabilities and data Accumulated advantages, quickly constructed the new coronavirus spike protein, and applied for a patent. Both BNT162b2 and mRNA-1273 applied NIH related patents.
In terms of mRNA delivery in vivo, the key technology is the ability to use lipid nanoparticles to deliver mRNA to cells. It was jointly completed in 1998 by the University of British Columbia (UBC) and Arbutus Biopharmaceuticals in Canada, and was later licensed to Moderna, BioNTech, and PROTIVA.
Although Professor Mario performed desensitization in the article and did not disclose key information such as the patent number, it does not matter. After a “long” search, the author still found some of the key patents. The following table shows the patent numbers and corresponding information of some patents.
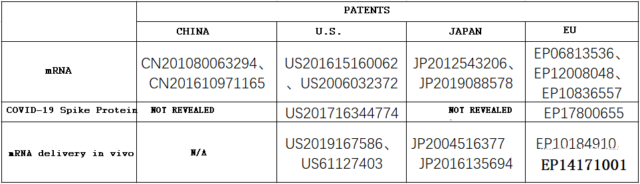
Other domestic and foreign companies such as Precision NanoSystems, which cooperated with CanSino for mRNA lipid nanoparticle vaccines, have also applied for related patents, so I won’t repeat them here.
Both Biontech and Moderna have gone through more than a dozen years of mRNA development, and they were unknown before the outbreak of COVID-19, but in the last more than a year, the stocks of both have risen more than ten times.
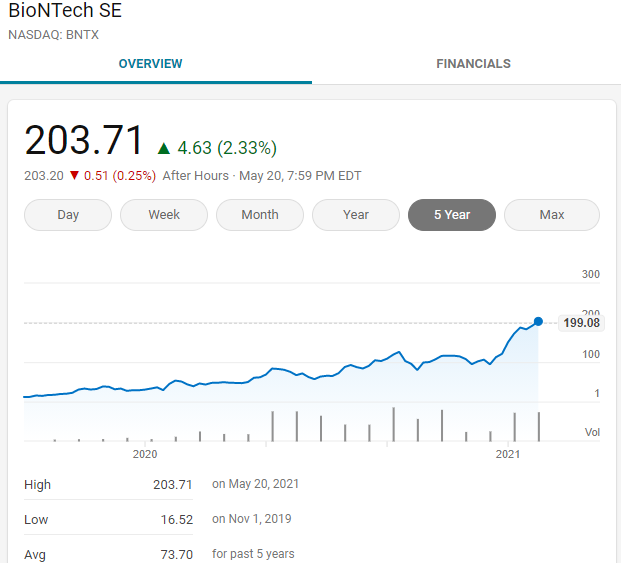
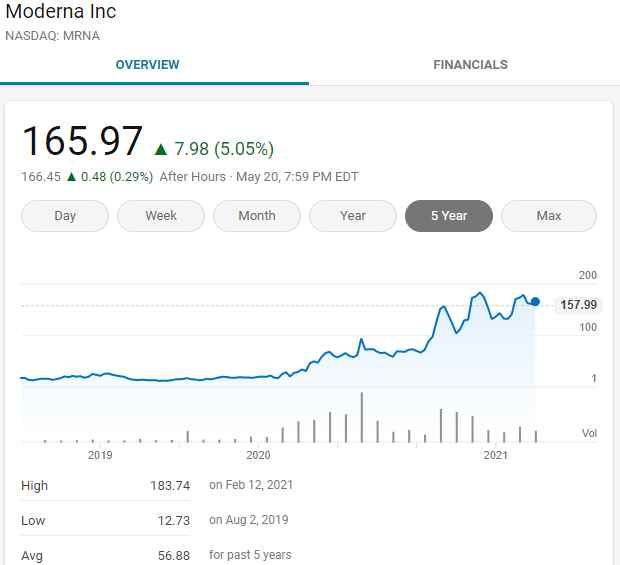
High returns always correspond to high risks. Innovative drug development is a vivid case after another. Of course, simply taking high risks will never be inevitable. Having a pair of discovering eyes is the key to increasing the success rate. We are determined in the right direction. Moving forward and seizing the opportunities that come are the core of success.
(source:internet, reference only)
Disclaimer of medicaltrend.org



