Combining stem cells to treat myocardial infarction
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Combining stem cells to treat myocardial infarction
Combining stem cells to treat myocardial infarction. Myocardial infarction irreversibly destroys millions of cardiomyocytes in the ventricle, making it the leading cause of heart failure worldwide.
In the past 20 years, many progenitor and stem cell types have been considered ideal candidates for regeneration after heart injury.
The potential of stem cell therapy has been thoroughly studied in animal and human studies, with the goal of repairing the heart through true tissue replacement, immune regulation, or through the secretion of paracrine factors to stimulate endogenous repair processes.
Although there have been some successful results in animal models, the results of clinical trials are still disappointing overall, mainly due to the limited stem cell survival and retention after transplantation.
A wide range of interests have developed the combined use of stem cells and various priming strategies to improve the efficacy of regenerative cell therapy.
In this review, the author has a key discussion on the different stem cell types studied in preclinical and clinical studies in the field of cardiac repair.
In addition, the author gives the latest progress in stem cell combination and pretreatment, and discusses the future prospects of these new regeneration strategies.

Image source: https://pubmed.ncbi.nlm.nih.gov/34114238/
Cardiovascular disease (CVD) has a great impact on the global society because it is the leading cause of death and disability worldwide. Myocardial infarction (MI) is a common clinical manifestation of CVD, which can quickly lead to heart failure by destroying about 25% of the ventricular tissue.
Due to the limited regenerative potential of the heart, heart damage such as myocardial infarction can cause an irreversible and huge loss of approximately 1 billion cardiomyocytes in the left ventricle. Although revascularization strategies and treatments are used to slow down ventricular remodeling, current treatments cannot replace lost heart tissue and therefore cannot prevent the progression of heart failure.
In the late stage of heart failure, heart transplantation is the only option for these patients, but mainly due to the shortage of organ donors and the risks of surgery, heart transplantation cannot be provided to all patients. The latter emphasizes the need for new strategies to regenerate lost heart tissue.
For a long time, it has been believed that the heart cannot regenerate the damaged myocardium after myocardial injury. At the beginning of this century, the concept of cardiomyocytes was discovered to proliferate in the mammalian heart. Although the replacement of cardiomyocytes occurs, the turnover rate of the heart is still less than 1% per year, which emphasizes the fact that endogenous heart regeneration is a rare event and not enough to repair millions of lost cardiomyocytes in infarcted tissue.
With the goal of preventing or treating heart failure, finding an ideal method to promote the regeneration of the damaged heart has aroused great interest among researchers and clinicians. Cell transplantation is one of the most intensively studied regeneration strategies in heart repair.
The potential of using stem cells or other cell types for cell therapy has been fully studied in animal research and clinical trials. Unfortunately, despite the results of some promising animal models, a successful stem cell therapy to regenerate injured heart tissue has not yet reached the clinic.
Generally speaking, there are two mechanisms for stem cell therapy to induce heart repair. The first mechanism involves the production of new cardiomyocytes after heart injury to truly replace the lost heart tissue.
This requires stem cells or progenitor cells (such as embryonic stem cells, induced pluripotent stem cells, and cardiac stem cells) to differentiate into functional cardiomyocytes and electromechanically coupled with the host myocardium.
Heart remuscularization may be the most intuitive strategy for restoring heart function after myocardial infarction, but this cannot be achieved by most stem cell types that have been studied because they do not differentiate and integrate into heart tissue.
The second mechanism involved in tissue repair depends on the specific characteristics of stem cells, that is, their ability to secrete multiple factors.
In fact, most stem cells are likely to promote heart repair through paracrine action.
Stem cells are a rich source of soluble factors and extracellular vesicles, which can regulate the endogenous repair process by stimulating angiogenesis, reducing fibrosis, increasing the survival rate of cardiomyocytes and affecting immune response.
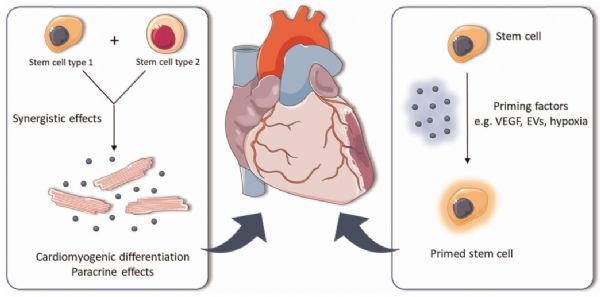
Strategies to strengthen heart repair after injury
Image source: https://pubmed.ncbi.nlm.nih.gov/34114238/
In this total, the author reviews the various stem cell types studied in the field of cardiac repair in the past two decades, focusing on their characteristics and their potential in preclinical and clinical research.
The author also discussed the combined use of these stem cell types in heart regeneration and explored the prospects of stem cell pretreatment.
Although the number of studies using one or two stem cell types to treat MI is still growing, this review aims to update current insights and analyze future directions.
(sourceinternet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



