How to choose a good target for CAR-T cells in Immunotherapy?
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
How to choose a good target for CAR-T cells in Immunotherapy?
How to choose a good target for CAR-T cells in Immunotherapy? Since 2017, the FDA has approved Kymriah and other 5 CAR-T cell products (see Table 1). In June of this year, China approved the CD19 target autologous CAR-T cell therapy product, Akirensai injection (product code FKC876). ) Listed.
Table 1 FDA approved cell therapy products for marketing
In view of the fact that CAR-T therapy basically has a mature CAR structure, production and preparation methods and clinical treatment plans, how to select and use targets has become the key to determining its potential. In this regard, there is no uniform target selection guideline, and the standards usually need to be modified according to actual clinical needs.
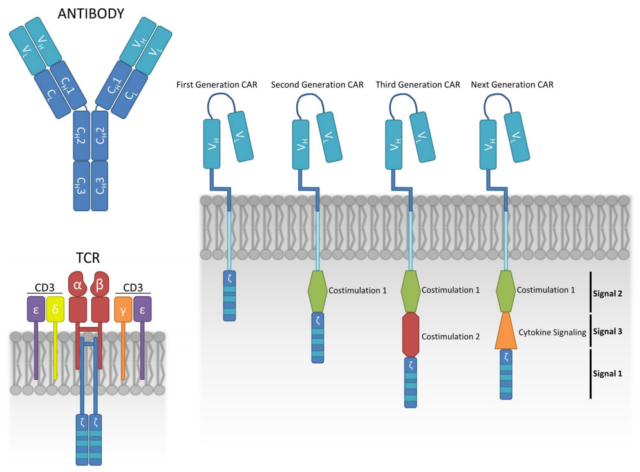
Figure 1 Design of chimeric antigen receptor (CAR)-T cell structure
1. Criteria for choosing a good target
High coverage
CAR molecules target the surface antigens of tumor cells. Not only proteins, but carbohydrates and glycolipid molecules may also be potential targets. The interaction between CAR and target leads to the formation of immune synapses, with which contact-dependent cytotoxicity occurs. In order to achieve significant tumor clearance, CAR-T cells should target most tumor cells, that is, the selected target antigen should have sufficient coverage on tumor cells.
At present, most CART therapies have good clinical efficacy and meet the selection criteria of high coverage, such as CD19, CD20 and B cell maturation antigen (BCMA).
High specificity
Tumor antigens include tumor-specific antigen (TSA) and tumor-associated antigen (TAA). TSA is only found on cancer cells, not healthy cells. The level of TAA is elevated on tumor cells, but the level of expression on healthy cells is also lower. The specificity of TSA will be significantly higher than that of TAA, but this antigen expressed only in tumor cells is very rare, which limits its application in CAR-T. The specificity of the selected target should be sufficient to prevent CAR-T cells from causing serious organ damage.
Stability
Due to the high evolutionary potential brought about by genome instability, cancer cells can quickly acquire a phenotype that prevents immune killing. In CAR-T therapy, loss of target is a very common mechanism for treatment failure. Both theory and experience have proved that the more unstable the target, the easier it is for cancer cells to escape from killing CAR-T cells. Therefore, as an ideal target, its expression should be fixed. If the expression is floating, the processing will be difficult to be effective.
Therefore, the ideal goal should be high coverage and high specificity to ensure effectiveness and safety. In addition, the stability of antigen expression is also very important. However, the “ideal” goal does not actually exist.
2. Typical Case
From the beginning of the marketed products (Table 1), CD19 and BCMA are currently the best and most mature targets used in CAR-T therapy.
At present, CD19 has been proven to be effective and safe in the treatment of B-ALL, chronic lymphocytic leukemia (CLL), mantle lymphoma (CML) and B-cell lymphoma (NHL). CD19 is widely and limitedly expressed throughout the entire stage of B cell development, until the final differentiation into plasma cells (Figure 1a). Therefore, CD19 has perfect coverage of B-cell malignant tumors, which enables CAR-T-19 treatment to obtain a high CRR.
However, in terms of specificity, CD19 is not an ideal target. With the anti-tumor effect of CAR-T-19, normal B cells will also be eliminated, leading to long-term B cell dysplasia. Fortunately, with effective clinical management, patients can tolerate B cell hypoplasia.

Figure 2 Schematic diagram of CD19 and CD22 expression
Take CD19 and CD22 as examples, their expression is almost the same (Figure 2a). From the perspective of coverage and specificity, it can be inferred that CAR-T-19 and CAR-T-22 therapy should have similar anti-tumor potential in the treatment of B-cell lymphoma. However, in clinical practice, CAR-T-19 therapy shows more significant and long-lasting anti-tumor activity. In patients who relapse after receiving CAR-T-19 or CAR-T-22 treatment, target loss is the most common cause.
BCMA is also similar. Although BCMA is not strictly expressed on tumor cells, due to its high coverage and tolerable off-target effects, these CAR-T treatments have shown excellent clinical prospects.

Figure 3 Schematic diagram of the expression of BCMA
Therefore, we believe that the coverage of single-target CAR-T therapy should be sufficiently high. In terms of its specificity, non-tumor effects need to be rigorously evaluated and tested. In the embodiment, the treatment intensity that needs to be set through an appropriate window is determined by the toxic side effects. When side effects outside the tumor can be tolerated, specificity may be reduced in practice.
3. Use other targets to overcome escape
In addition to CD19 and BCMA, the clinical trials of CAR-T cell therapy target hematological malignancies, as well as CD22, CD20, CD138, CD33, CD123, inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1), Immunoglobulin κ chain (Igκ) and Lewis Y antigen (Table 2 and Figure 4).
To overcome the problem of such antigen targets regarding escape variants, one approach is to study other tumor antigen targets, for example. Another approach is to develop new strategies for designing CAR-T cells, such as bispecific chimeric antigen receptors. For example, the design of CD19/CD20 tandem CAR-T cells can effectively kill tumor cells when encountering any kind of antigen.
Table 2: Some CAR-T cell targets used to treat hematological tumors

Figure 4 CAR T cells currently in clinical trials
Summary
Coverage is the main factor to be considered, it directly determines the upper limit of CAR-T treatment, and specificity is also a basic factor to be considered. It can affect the effectiveness of CAR-T treatment by affecting the intensity of treatment. In addition, the expression of ideal goals must be fixed. Otherwise, rapid and frequent target loss will lead to CAR-T treatment failure.
For the treatment of solid tumors, it is difficult to obtain ideal targets such as CD19 and BCMA, and it is almost impossible to obtain targets with sufficient coverage. In addition, most of the targets currently tested will bring obvious off-target effects, so the treatment intensity is usually limited, which further weakens the efficacy.
Therefore, the treatment of solid tumors may need to combine multiple targets and endogenous anti-tumor effects, such as activating the endogenous tumor immune response and destroying the tumor growth environment.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



